Advanced Search
TcGDT1-3xHA overexpression
Last updated date: Sep 17, 2022 Views: 569 Forks: 0
(from PLoS Path: Deletion of a Golgi protein in Trypanosoma cruzi reveals a critical role for Mn2+ in protein glycosylation needed for host cell invasion and intracellular replication
DOI: 10.1371/journal.ppat.1009399
1) T. cruzi epimastigotes RNA was purified using Trizol method. See manufacturers protocol here – https://assets.thermofisher.com/TFS-Assets/LSG/manuals/trizol_reagent.pdf
2) RNA was used for cDNA synthesis using the first strand cDNA synthesis kit. See manufacturer’s protocol here - https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FsuperscriptIIIfirststrand_pps.pdf
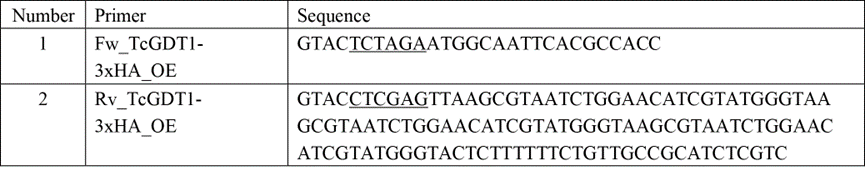
3) cDNA was quantified using nanodrop and 50ng were used for a PCR reaction along with primers 1 and 2 from table below, following the manufacturers protocol for Q5 high fidelity DNA polymerase. See manufacturers protocol here - https://www.neb.com/protocols/2013/12/13/pcr-using-q5-high-fidelity-dna-polymerase-m0491
4) The PCR product was purified using PCR purification kit and following manufacturers protocol described here - https://files.zymoresearch.com/protocols/_d4005_d4006__d4033_d4034_dna_clean_concentrator-25_kit.pdf
5) Purified PCR product and pTREX-n plasmid were digested with restriction enzymes XbaI/XhoI from NEB in a 20µL reaction.
6) The digested PCR product and digested plasmid were separated using agarose gel electrophoresis using 0.8% agarose gel and purified using gel purification kit using manufacturer’s protocol described here - https://files.zymoresearch.com/protocols/_d4001t_d4001_d4002_d4007_d4008_zymoclean_gel_dna_recovery_kit.pdf
7) Following purification, the PCR product was ligated into the purified plasmid using T4 DNA ligase from Promega using manufacturer’s protocol - https://www.promega.com/-/media/files/resources/protocols/product-information-sheets/g/t4-dna-ligase-blue-white-cloning-qualified-protocol.pdf
8) The ligated product was transformed into chemically competent E. coli from NEB (https://www.neb.com/products/c2987-neb-5-alpha-competent-e-coli-high-efficiency#Product%20Information) using heat shock method. See protocol here. - https://www.goldbio.com/documents/4066/DH5-alpha%20Chemically%20Competent%20E.%20coli%20cells%20Transformation%20Protocol.pdf
9) Transformed cells were selected by growth at 37 degrees Celsius for 18 hours on Luria Bertani nutrient agar plate with 100 µg Ampicillin.
10) Transformed colonies were confirmed for TcGDT1 containing plasmid using a colony screen PCR with Primers 1 and 2 from table above. See colony PCR protocol here - https://protocols.scienceexchange.com/protocols/colony-screening-by-pcr
11) The colonies that carried the transformed plasmid were grown in LB broth with 100 µg ampicillin overnight and plasmid DNA was extracted using DNA extraction kit using manufacturer’s protocol - https://files.zymoresearch.com/protocols/_d4208t_d4209_d4210_d4211_d4212_zymopure_plasmid_miniprep.pdf
12) The extracted DNA was quantified using nanodrop and sequenced using primers 1 and 2 from table above with Genewiz (Azenta) sequencing service (https://www.genewiz.com/en/Public/Resources/Sample-Submission-Guidelines/Sanger-Sequencing-Sample-Submission-Guidelines/Sample-Preparation#sanger-sequence)
13) After confirming the sequence, a larger bacterial culture was prepared and DNA was extracted using manufacturers protocol - https://files.zymoresearch.com/protocols/_d4200_d4201_zymopure_ii_plasmid_midiprep.pdf
14) The purified DNA was electroporated using our laboratory protocol described in APPENDIX I of this document.
15) A stable parasite population was selected using 250 µg/mL of G418 (took about 2-3 weeks).
16) Drug resistant parasites were processed for immunofluorescence analysis using our laboratory protocol described in APPENDIX II of this document.
Appendix I
Electroporation of T. cruzi epimastigotes
(Docampo-Moreno lab, modified from David Engman’s lab protocol)
1. Grow T. cruzi epimastigotes to a mid-log stage at density 1-2 x 107 cells/mL.
2. Wash cells once in PBS pH 7.4.
3. Resuspend cells at a density of 1 x 108 cells/mL in electroporation buffer(recipe below).
4. Mix 0.4 mL of cell suspension with 10 μL DNA (10, 25, 50 μg)* in ice cold 0.4 cm electroporation cuvettes** so that final volume is 410 μL. As controls form the electroporation, add 10 μL of sterile water to one cuvette of cells, and add nothing to another cuvette of cells.
5. Keep cells on ice for 10 min.
6. Electroporate using Bio-Rad gene pulser set at 1.5 kV and 25 μF with 3 pulses (allow for 10 sec between pulses). Expected time constants: between 0.9-1.0 ms.
7. Let cells recover in cuvette at RT for approximately 15 min.
8. Transfer cells to 5 mL LIT media supplemented with 20% FBS (heat inactivated) and incubate at 28°C.
9. After 24 h, add antibiotic for selection (G418 or Hygromycin, 250 μg/mL).
Electroporation buffer 1 L of Electroporation buffer
120 mM KCl (MW=74.55 g/mol) 8.95 g KCl
0.15 mM CaCl2 (MW=110.99 g/mol) 0.02 g CaCl2
10 mM K2HPO4 (MW=174.20 g/mol) 1.74 g K2HPO4
25 mM HEPES (MW=238.30 g/mol) 5.96 g HEPES
2 mM EDTA (MW=372.24 g/mol) 0.77 g EDTA
5 mM MgCl2 (MW=95.21 g/mol) 0.48 g MgCl2
Adjust pH to 7.6 and filter sterilize, then storage at 4°C.
* We have obtained better results when using 25 and 50 μg DNA.
**Alternative electroporation conditions: 0.2 cm cuvettes, 0.3 kV, 500 μF, however,
we have obtained higher transfection efficiencies when using 0.4 cm cuvettes at 1.5
kV and 25 μF.
Appendix II
1) Epimastigotes were washed with PBS (pH 7.4) and fixed with 4% paraformaldehyde in PBS (pH 7.4) for 1 h, at RT.
2) Cells were adhered to poly-L-lysine coated coverslips and then permeabilized with 0.1% Triton X-100 for 5 min.
3) Permeabilized cells were blocked with PBS (pH 7.4) containing 3% BSA, 1% fish gelatin, 50 mM NH4Cl, and 5% goat serum overnight at 4°C.
4) Then, cells were incubated with a primary antibody [rat anti-HA-Tag (1:500) or monoclonal mouse anti-AP1γ 211.F7 (1:80)], diluted in 1% BSA in PBS (pH 8.0) for 1 h, at RT.
5) Cells were washed three times with 1% BSA in PBS (pH 8.0), and then incubated for 1 h, at RT in the dark with Alexa Fluor 488 –conjugated goat anti-rat secondary antibody (1:1,000) or Alexa Fluor 546 –conjugated goat anti-mouse secondary antibody (1:1,000).
6) Then, cells were washed and mounted on slides using Fluoromount-G mounting medium containing 5 μg/ml of 4’,6- diamidino-2-phenylindole (DAPI) to stain DNA.
7) Controls were performed as described above but in the absence of a primary antibody.
8) Differential interference contrast and fluorescent optical images were acquired with a 100× objective (1.35 aperture) under non-saturating conditions with a Photometrix CoolSnapHQ charge-coupled device camera driven by DeltaVision software (Applied Precision, Issaquah, WA) and deconvolved for 15 cycles using Softworx deconvolution software.
- Ramakrishnan, S and Docampo, R(2022). TcGDT1-3xHA overexpression. Bio-protocol Preprint. bio-protocol.org/prep1949.
- Ramakrishnan, S., Unger, L. M., Baptista, R. P., Cruz-Bustos, T. and Docampo, R.(2021). Deletion of a Golgi protein in Trypanosoma cruzi reveals a critical role for Mn2+ in protein glycosylation needed for host cell invasion and intracellular replication. PLoS Pathogens 17(3). DOI: 10.1371/journal.ppat.1009399
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

