Advanced Search
Total RNA extraction and quantitative RT-PCR
Last updated date: Sep 13, 2022 Views: 664 Forks: 0
Detailed working flow
Tissues or culture cells sample preparations:
1. For culture cells, put the cell culture dish on ice (A), remove the medium and wash with cold PBS (B), add TRI Reagent (for example, 0.5 ml of TRI Reagent/6 cm dish) and put the dish on a shaker and shake for 5 min at ambient temperature. Scrape the solution to one edge of the dish (C), pipetting the sticky solution against the bottom of the dish for 20 strokes to shear the thick mixture (D), transfer the solution into an Eppendorf microtube (E). Rotate the tube for 20 min at 4 °C and centrifuge it at 1500 rpm (21,000 g), 4 °C for 15 min (F). Finally collect the upper layer solution into a new tube, and proceed to RNA extraction

2. For mouse tissues:
A. For tissues less than 40 mg, collect the tissue directly into the RINO 1.5 ml screw cap tube (A) that contains TRI Reagent (1 mg tissue to 10 µL TRI Reagent) and one spoon of the zirconium oxide beads (RNase-free), do beads homogenization at the max speed for 5 min using the Bullet Blender Storm Pro (1.5 mL screw and snap cap tubes) (B). Centrifuge the tube at 1500 rpm (21,000 g), 4 °C for 15 min, collect the supernatant solution into a new tube, and proceed to RNA extraction
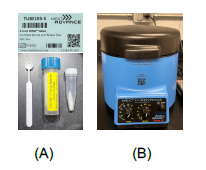
B. For large pieces of tissues or whole organ:
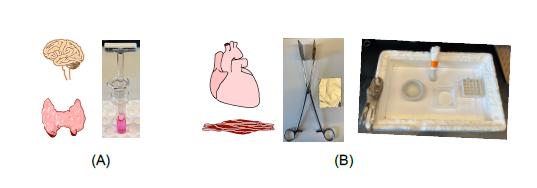
a) For soft tissue, e.g. brain and adipose tissue, collect fresh tissues and add into the Dounce glass homogenizer containing 10X volume of TRI reagent and homogenize for 15 stokes (A). Transfer the lysates into a proper tube, centrifuge at 1500 rpm (21,000 g), 4 °C for 15 min, remove the top transparent layer (containing lipids) and then collect the upper layer solution into a new tube, and proceed to RNA extraction.
Note: for liver tissue, homogenize the tissue in TRI Reagent in a ratio of 1 mg to 25 µL.
b) For skeletal muscle or heart, snap freeze the tissue using a modified clamp that is pre-chilled in liquid nitrogen, wrap the flattened tissue with labeled foil and put it into liquid nitrogen (B). Crush the frozen tissue in a mortar with pestle in liquid nitrogen (B), scrape the tissue powder using a disposable plastic lab spatula and grab the mortar using a curved jaw locking plier to transfer the tissue powder into a disposable plastic weighing tray, bend the tray to gather the powder together onto one corner of the tray, and pour the powder into a 1.5 ml RINO tube prechilled in liquid nitrogen (B). Take the tissue powder up to 40 mg into the RINO 1.5 ml screw cap tube that contains TRI Reagent (1 mg tissue powder to 10 µL TRI Reagent) and one spoon of the zirconium oxide beads (RNase-free), do beads homogenization at the max speed for 5 min using the Bullet Blender Storm Pro (1.5 mL screw and snap cap tubes) (B). Centrifuge the tube at 1500 rpm (21,000 g), 4 °C for 15 min, collect the supernatant solution into a new tube, and proceed to RNA extraction
Total RNA was extracted using TRI reagent and Direct-zol RNA MiniPrep Plus kit (Zymo Research):
RNA Purification. Perform all steps at room temperature and centrifugation at 10,000-16,000 x g for 30 seconds, unless specified.
1. Add an equal volume ethanol (95-100%) to a sample lysed in TRI Reagent® or similar reagents and mix thoroughly.
2. Transfer the mixture into a Zymo-Spin™ IICR Column in a Collection Tube and centrifuge. Transfer the column into a new collection tube and discard the flow-through.
3. DNase I treatment (recommended)
(D1) Add 400 µL RNA Wash Buffer to the column and centrifuge.
(D2) In an RNase-free tube (not included), add 5 µl DNase I (6 U/µL), 75 µL DNA Digestion Buffer and mix by gentle inversion. Add the mix directly to the column matrix.
(D3) Incubate at room temperature (20-30°C) for 15 minutes. Proceed to step 4.
4. Add 400 µL Direct-zol™ RNA PreWash5 to the column and centrifuge. Discard the flow- through and repeat this step.
5. Add 700 µL RNA Wash Buffer to the column and centrifuge for 1 minute to ensure complete removal of the wash buffer. Transfer the column carefully into an RNase-free tube (not included).
6. To elute RNA, add 50 µL of DNase/RNase-Free Water directly to the column matrix and centrifuge. Alternatively, for highly concentrated RNA use ≥ 25 µL elution. The eluted RNA can be used immediately or stored frozen.
Note:
Measure the RNA concentration using Nanodrop and the value of 280/260 and A230/A260 should be around 2.0

First-strand cDNA was synthesized using the SuperScript IV VILO cDNA synthesis kit (ThermoFisher Scientific):
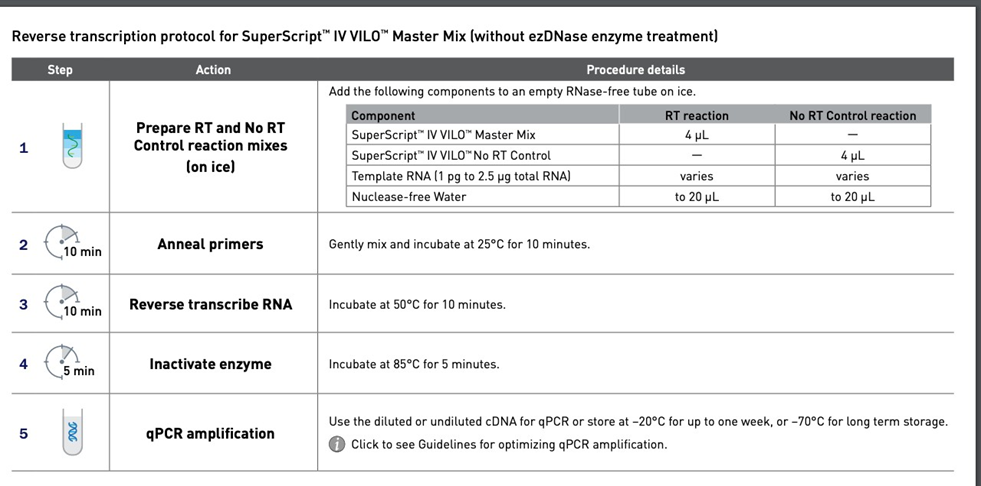
Note:
1. Take 100 to 500 ng of the total RNA to perform cDNA synthesis according to manual of Super IV VILO kit.
2. For the subsequent qPCR reaction, dilute the 20 µL reaction solution with extra-pure water to 400 µL, mix gently, and spin down.

TaqMan RT-qPCR was performed for the measurement of mRNA using the ΔΔCt method:
1. Set up the QuantStudio 6 Flex Real-Time System machine, define the well target and sample design
2. Add 10 uL of reaction mixture (2.3 ul of H2O, 7 uL of 2X master solution (B), and 0.7 uL of 20X primer and probe solution (C) into a well of the 384-well plate (D), add 4 uL of cDNA solution into the well (change the pipette tip for each well).
3. Seal the plate with adhesive film firmly and vortex the plate for 10 seconds, spin down the solution using a microplate centrifuge.
4. Perform the qPCR reaction and export the analyzed data to excel. Use Prism 8 or other software for data statistical analysis and graph production.

List for Main Reagents and Instruments

Note: The images of brain, adipose tissue, heart, and muscle are from the template image of the software ChemDraw 20.1. All the other images are from the laboratory of Dr. Jeffery E. Pessin.
- Tang, Y and Pessin, J(2022). Total RNA extraction and quantitative RT-PCR. Bio-protocol Preprint. bio-protocol.org/prep1924.
- Tang, Y., Zong, H., Kwon, H., Qiu, Y., Pessin, J. B., Wu, L., Buddo, K. A., Boykov, I., Schmidt, C. A., Lin, C., Neufer, P. D., Schwartz, G. J., Kurland, I. J. and Pessin, J.(2022). TIGAR deficiency enhances skeletal muscle thermogenesis by increasing neuromuscular junction cholinergic signaling. eLife. DOI: 10.7554/eLife.73360
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
