Advanced Search
The luciferase reporter Sec UGA read through assay
Last updated date: Aug 8, 2022 Views: 569 Forks: 0
Abstract. The synthesis of eukaryotic selenoproteins is reliant on a mechanism of elongation where in-frame UGA STOP codons are interpreted as selenocysteine (Sec). This protocol for monitoring elongation of a eukaryotic selenoprotein at an in-frame UGA codon employs a luciferase reporter based readthrough assay.
Graphic abstract:
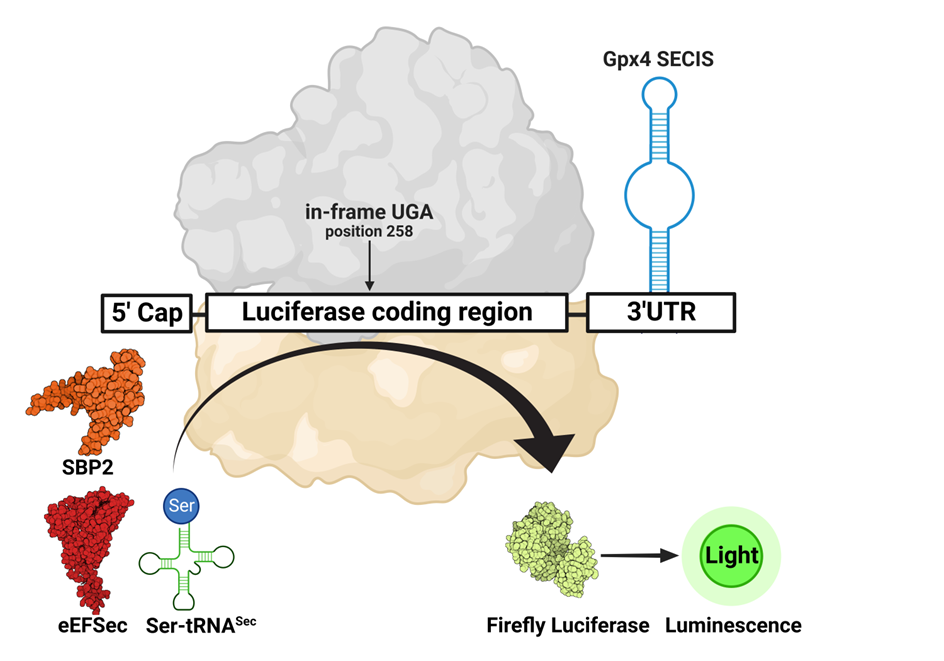
Keywords: selenocysteine, UGA, codon suppression, recoding, luciferase, reporter assay
Background. Suppression of an in-frame UGA codon is achieved through a complex of the 80S ribosome, mRNA that carries in its 3’-untranslated region (UTR) a Sec insertion sequence (SECIS), SECIS-binding protein 2 (SBP2), aminoacylated tRNASec and Sec-specific eukaryotic elongation factor eEFSec. The assay was adapted from Gupta et al. 2013 for use in our published study entitled “Structure of the mammalian ribosome as it decodes the selenocysteine UGA codon” to test the incorporation of Ser-tRNASec at the SEC UGA codon. This assay was further expanded to analyze a series of mutants of SBP2 and eEFSec.
Materials and Reagents
- Wheat germ extract Promega CAT# L4380
- Complete amino acid mix 1mM Promega CAT# L4461
- Luciferase assay system Promega CAT# E1500
- Ser-tRNASec was prepared as described in (Holman et al. 2017)
- mRNA was prepared as described in (Gupta et al. 2013)
- eEfSec was prepared as described in (Dobosz-Bartoszek et al. 2016)
- SBP2 was prepared as described in (Hilal et al. 2022)
- PBS buffer see recipes
Equipment
- Falcon 96-well TC plates, white CAT# 08-771
- BMG FLUOstar Omega 96-well plate luminometer (BMG Labtech, Inc., USA)
Software
- Graphpad prism 9.0, Dotmatics, https://www.graphpad.com/
Procedure
Reaction content
| Component | Vol (µL) |
| Wheat Germ Lysate | 6.25 |
| Complete amino acid mix | 0.25 |
| mRNA 200 ng/ µl | 1 |
| Ser-tRNASec 38µM (Nuclease free water) | 2 |
| eEFSec 8 µM (50 mM Hepes pH 7.5, 150 mM NaCl, 5mM MgCl2, 0.5 mM TCEP) | 0.5 |
| SBP2 8 µM (10 mM Tris pH 8.0, 300 mM NaCl, 5% (v/v) glycerol, 0.5 mM TCEP) | 0.5 |
| Water (Nuclease free water) | 2 |
| Total | 12.5 |
*Very important. Aminoacylated tRNA must be desalted before adding to the mixture and must be used immediately.
- At room temperature, combine components for each reaction in 1.5mL tubes and gently mix by pipetting up and down.
- Incubate reactions at room temperature (~ +21-23°C) for 2 hours.
- Transfer reactions onto 96-well TC plate
- Dilute each reaction with 37.5 µL of PBS, pH 7.
- Place plate into BMG FLUOstar Omega 96-well plate luminometer
- Prepare luciferase substrate in accordance with provided kit instructions
- Prior to each reading, inject 100 µL of luciferase substrate via luminometer injector.
- After 2 second delay, read each well for 10 seconds.
- Luminescence is usually weak and may require adjusting the readers gain.
- Export data and analyze in GraphPad prism 9.0.
Data analysis
Data was exported from the luminometer as an excel spreadsheet and the data was transferred to GraphPad Prism 9.0 for analysis and graphing.
Note
Variability can be quite high with this experiment especially when using lysate. Luminescence is comparatively low to the kit provided positive control and gain will likely need to be adjusted for optimal results. Ser-tRNASec should be used immediately after aminoacylation as it quickly degrades. Ensure that the aminoacylation reaction has been desalted and replaced with nuclease free water to avoid a severe loss in readthrough. Unacylated tRNASec can also yield some readthrough due to charging by lysate SerRS.
Recipes
- Reaction contents outlined in procedure
- Phosphate buffer saline (PBS) pH 7.4 0.137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4,1.76 mM KH2PO4.
- See (Gupta et al 2013) for preparation of mRNA.
- See (Dobosz-Bartoszek et al. 2016) for preparation of eEFSec
- See (Hilal et al. 2022) for preparation of SBP2
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIGMS GM097042 to MS and GM077073 to PRC and MS), ACS-IL (225752 to MS) and UIC Center for Clinical and Translational Sciences (to MDB).
This work was in part supported by The National Institute of General Medical Sciences, National Institutes of Health grant GM097042 (to MS). This article was prepared while MS was employed at the University of Illinois at Chicago. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Special thanks to Paul R. Copeland for the original assay this was adapted from in addition to support and guidance with the luciferase reporter Sec UGA read through assay.
Competing interests
The authors declare no competing interests
References
Dobosz-Bartoszek, M., Pinkerton, M. H., Otwinowski, Z., Chakravarthy, S., Söll, D., Copeland, P. R., & Simonović, M. (2016). Crystal structures of the human elongation factor eefsec suggest a non-canonical mechanism for Selenocysteine incorporation. Nature Communications, 7(1). https://doi.org/10.1038/ncomms12941
Gupta, N., DeMong, L. W., Banda, S., & Copeland, P. R. (2013). Reconstitution of selenocysteine incorporation reveals intrinsic regulation by Secis Elements. Journal of Molecular Biology, 425(14), 2415–2422. https://doi.org/10.1016/j.jmb.2013.04.016
Hilal, T., Killam, B. Y., Grozdanović, M., Dobosz-Bartoszek, M., Loerke, J., Bürger, J., Mielke, T., Copeland, P. R., Simonović, M., & Spahn, C. M. (2022). Structure of the mammalian ribosome as it decodes the selenocysteine UGA Codon. Science, 376(6599), 1338–1343. https://doi.org/10.1126/science.abg3875
Holman, K. M., Puppala, A. K., Lee, J. W., Lee, H., & Simonović, M. (2017). Insights into substrate promiscuity of human seryl-trna synthetase. RNA, 23(11), 1685–1699. https://doi.org/10.1261/rna.061069.117
- Killam, B Y, Copeland, P R and Simonović, M(2022). The luciferase reporter Sec UGA read through assay. Bio-protocol Preprint. bio-protocol.org/prep1849.
- Hilal, T., Killam, B. Y., Grozdanović, M., Dobosz-Bartoszek, M., Loerke, J., Bürger, J., Mielke, T., Copeland, P. R., Simonović, M. and Spahn, C. M. T.(2022). Structure of the mammalian ribosome as it decodes the selenocysteine UGA codon. Science 376(6599). DOI: 10.1126/science.abg3875
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
