Advanced Search
Hematoxylin & Eosin (H&E) staining
Last updated date: Jul 29, 2022 Views: 680 Forks: 0
Hematoxylin & Eosin (H&E) staining of skeletal myotubes and skeletal muscle tissues
Hyun-Jun Kim1, Da-Woon Jung1*, and Darren R. Williams1*
1New Drug Targets Laboratory, School of Life Sciences, Gwangju Institute of Science and Technology, 123 Cheomdangwagi-ro, Buk-Gu, Gwangju 61005, Republic of Korea
*Correspondence: 1) darren@gist.ac.kr, 2) jung@gist.ac.kr.
Technical note: Please note that this protocol was developed for staining skeletal myotube cultures. We have not tested this protocol with other cell types. In addition, we noticed a difference in the staining intensity for human and murine myotubes: human primary myotube cultures stained more faintly for eosin in the cytoplasm compared to C2C12 myotubes. Additionally, for H&E staining of skeletal muscle, we include a protocol describing staining of the gastrocnemius muscle.
Detailed protocol for H&E staining of murine C2C12 myotubes and myotubes derived from human skeletal myoblasts (HSKM)
- Aspirate media and wash the cells with PBS for 2 times before fixation
- Fixation : treat 3.7 % formaldehyde solution on the cells at room temperature (RT) for 15 min
- Wash the cells with PBS for 3 times to remove residual formaldehyde solution
- Permeabilization : treat 0.2 % Triton X-100 solution at room temperature (RT) for 10 min
- Wash the cells with PBS for 3 times to remove residual permeabilization solution
- Wash the cells with deionized water for 2 times
- Treat Gill No.3 Hematoxylin solution for at room temperature (RT) for 1 min
- Wash the cells with tap water for 3 times
- Treat Eosin Y solution at room temperature (RT) for 45 sec
- Dehydration : wash the cells with 95 % and 100 % ethanol solution for 2 times on each step ( 95 % and 100 % ethanol were used to remove eosin solution )
- Immerse the cells in PBS before taking pictures with microscopy
Detailed protocol for H&E staining in gastrocnemius muscle
- Dissect out gastrocnemius muscle tissues from mice
- Sucrose embedding : continuously incubate gastrocnemius muscle tissues in 10 %, 20 % and 30 % of sucrose solution at 4℃ for 24 h each step
- Fixation : embed muscle tissues in 3.7 % of formaldehyde solution at 4℃ for 24 h
- Cryo-OCT embedding : embed muscle tissues in OCT solution to make Cryo-block
(it’s important to make the muscle tissues upright to get accurate size of CSA) - Cryo-section : section the slides at 8 µm of thickness
- Rinse the slides in deionized water for 1 min
- Incubate the slides in chamber containing Gill No.3 Hematoxylin solution for 1 min to stain the nuclei dark blue
- Rinse the stained slides with running tap water for 10 min
- Incubate the slides in chamber containing Eosin Y solution for 3 min
- Rinse the stained slides with deionized water for 2 min for 3 times to remove excess of Eosin solution
- Dehydration : incubate the slides in 95 % and 100 % ethanol solution for 2 min for 2 times respectively
- Incubate the slides in xylene for 2 min
- Cover the slides with Permount and coverslip
Materials and reagents
Product | Catalog # | Company |
Hematoxylin | 1.05174.0500 | Merck |
Eosin Y | 09859-500 | Polyscience |
Formaldehyde | 252549 | Merck |
Triton X-100 | T8787 | Merck |
Results
Figure 1. H&E staining on C2C12 myotubes (A) and HSKM myotubes (B)
|
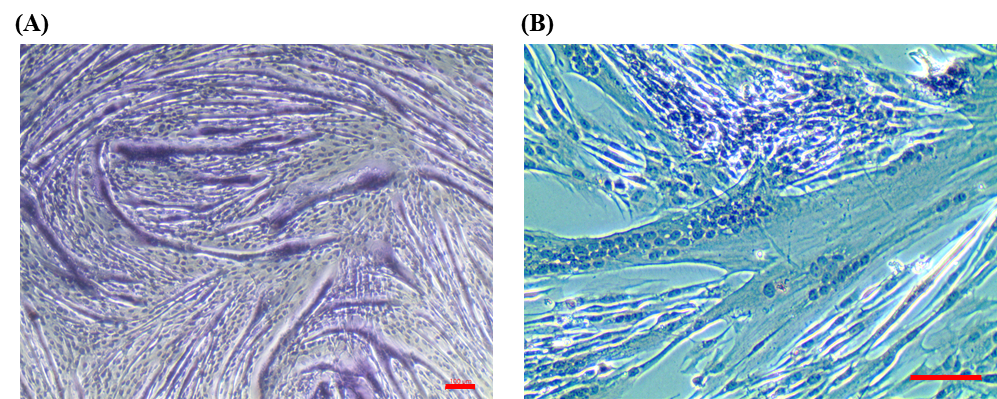
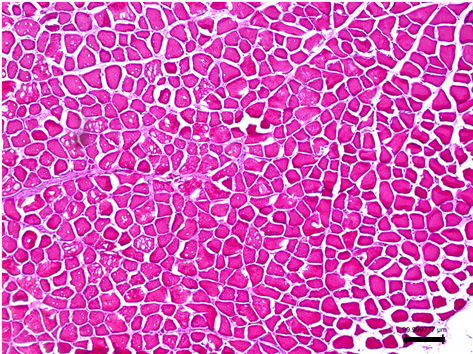
Figure 2. H&E staining on gastrocnemius muscle tissues
|
Related files
 Bio-protocol - H&E - Kim Jung Williams.docx
Bio-protocol - H&E - Kim Jung Williams.docx - Kim, H, Jung, D and Williams, D(2022). Hematoxylin & Eosin (H&E) staining. Bio-protocol Preprint. bio-protocol.org/prep1826.
- Kim, H., Lee, J., Kim, S., Lee, S., Jung, D. and Williams, D. R.(2021). Investigation of niclosamide as a repurposing agent for skeletal muscle atrophy. PLoS ONE 16(5). DOI: 10.1371/journal.pone.0252135
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
