Advanced Search
IP accumulation assay
Last updated date: Jul 22, 2022 Views: 781 Forks: 0
IP accumulation assay in transiently transfected cells, using CisBio IPOne commercial system in 384 WP format
This assay protocol is a variation of the standard protocol provided by the manufacturer (CisBio IP-One Gq HTRF, #62IPAPEB). Since NPY receptors do not couple naturally to the Gq pathway, cells are co-transfected with a chimeric GαΔ6qi4myr protein (kind gift of Evi Kostenis, Bonn University, Germany, details and sequence information in ref. 1) that redirects signaling from Gi/o to Gq. The utility of this procedure and the sensitivity of the resulting cellular system has been validated previously for NPY receptors (ref. 2). The assay is also useful to test the activity of receptor antagonists (ref. 3).
We have validated the protocol using HEK293, HEK293F and COS7 cells and using different commercial transfection reagents: (MetafectenePro (Biontex), Lipofectamine2000 (Invitrogen), PEI-MAXI 4000 (Polysciences), all according to their respective manufacturer's standard protocol. Full expression is reached ~36-48 h after transfection, so peptide stimulation should not yet be performed on day 3.
The assay is possible in adherent and solution-based protocol. While the adherent protocol is a little more laborious, it gives somewhat more control and measures activity in preferred state for adherent cell lines.
Step-by-Step-protocol, exemplified for HEK293:
Day 1:
Seed cells, e.g. 1.2 x 106 HEK293 in 6-well plates (A=9.6 cm² per well) for transfection at ~70-80% confluence on day 2.
Day 2:
Verify that cell confluence is about 70-80%. Perform transfection according to manufacturer's protocol. Use a total of 4 μg DNA per 10 cm² cell area, in a ratio of 4:1 receptor: GαΔ6qi4myr (e.g., 3200 ng plasmid encoding receptor, 800 ng plasmid encoding. It is critical to maintain this DNA ratio for good assay sensitivity without generating a large receptor reserve.
Day 3:
For adherent protocol: Wash cells once with Ca++/Mg++ - free PBS (1 ml per 10 cm2), detach with 0.2 ml Trypsin/EDTA and take up in full medium (DMEM/F12 + 15% FCS) to a concentration of 6.67 x 105 cells/ml. Re-suspend well and transfer 20.000 cells (30 μl) into an opaque 384-well plate (e.g., Greiner Bio One #781080). Return to the incubator for additional 20-24 h. Note: cell number needs to be adjusted if linear range of the assay is not met (see day 4).
For suspension protocol: Let cells grow another 24 h.
Day 4:
Adherent protocol: Prepare peptide dilution series in HBSS supplemented with 20 mM LiCl. LiCl inhibits the degradation of inositol monophosphate (IP1) to myo-inositol. Use low protein-binding reaction tubes (e.g. Eppendorf #0030108116) and/or low-binding binding compound plates (e.g. Greiner Bio One #655901) to avoid nonspecific binding of peptide to the plastics.
Discard supernatant by flipping the plate 3 times. Add 14 μl of stimulation solution preferably with an electronic 8-well dispensing micropipette from the compound plate according to the scheme below. Incubate 90 min @37°C.
Suspension protocol: Prepare peptide dilution series 1x StimB (from CisBio kit). As peptide solutions will be diluted 2-fold in the well, prepare for instance 2 x 10-12 M – 2 x 10-6 M if aiming to test 1 pM – 1 μM concentration range. CisBio’s StimB contains LiCl to inhibit the degradation of inositol monophosphate (IP1) to myo-inositol. Use low protein-binding reaction tubes (e.g. Eppendorf #0030108116) and/or low-binding binding compound plates (e.g. Greiner Bio One #655901) to avoid nonspecific binding of peptide to the plastics.
Harvest cells as described for adherent protocol on day 3, but adjust to 2.85 x 106 cells/ml. Optional: Pellet cells by centrifugation 150 x g for 3-5 min and re-suspend in stimulation buffer (1x, 5x stock provided in CisBio kit). Dispense 20.000 cells (7 μl) into an opaque 384-well plate (e.g., Greiner Bio One #781080). Add 7 μl of peptide dilutions (2x Stock) preferably with an electronic 8-well micropipette from the compound plate according to the scheme below. Incubate 90 min @37°C.
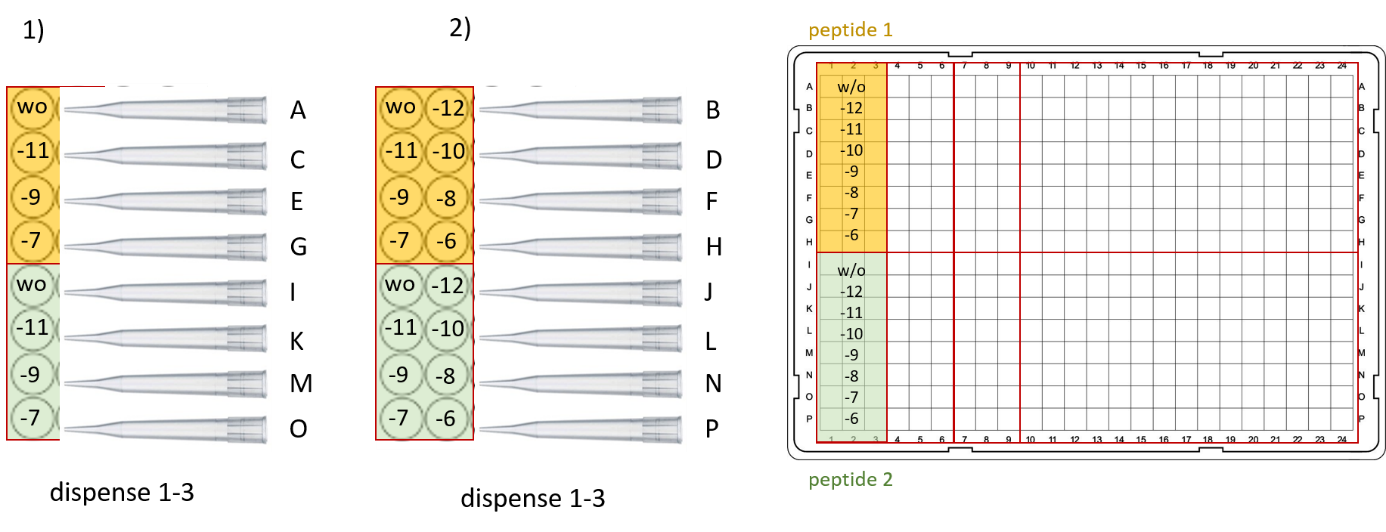
Figure 1: Suggested stimulation scheme for day 4. Left, 96-well compound plate with two different peptide dilution series (yellow and green, concentration indicated in log / M). Right, final plate layout in 384-WP.
Cell Lysis (adherent and solution protocol):
Prepare IP standard solutions according to the manual and dispense into empty wells in the 384 WP (not containing any cells or medium).
To all cell samples and IP Standard curve, add 3 μl of IP1-d2 working solution (diluted in cell lysis buffer) using a multi-dispenser pipette. Optional: spin down plate briefly in a swing-out rotor. Add 3 μl of anti-IP1-Tb Cryptate working solution (diluted in lysis buffer) using a multi-dispenser pipette. Optional: spin down plate briefly in a swing-out rotor. Incubate 1 h @ room temperature in the dark.
Measure in HTRF-compatible reader (e.g., Tecan Spark) at 320(exc)/620(em) and 320(exc)/665(em) and calculate the HTRF ratio. Make sure the assay falls into the linear range of detection determined by IP standard dilution series, otherwise adjust cell number on day 3.
References:
- E. Kostenis, Is Gα16 the optimal tool for fishing ligands of orphan G-protein-coupled receptors? Trends Pharmacol. Sci. 22, 560–564 (2001).
- Z. Yang, S. Han, M. Keller, A. Kaiser, B. J. Bender, M. Bosse, K. Burkert, L. M. Kögler, D. Wifling, G. Bernhardt, N. Plank, T. Littmann, P. Schmidt, C. Yi, B. Li, S. Ye, R. Zhang, B. Xu, D. Larhammar, R. C. Stevens, D. Huster, J. Meiler, Q. Zhao, A. G. Beck-Sickinger, A. Buschauer, B. Wu, Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor. Nature 556, 520–524 (2018).
- T. Tang, C. Hartig, Q. Chen, W. Zhao, A. Kaiser, X. Zhang, H. Zhang, H. Qu, C. Yi, L. Ma, S. Han, Q. Zhao, A. G. Beck-Sickinger, B. Wu, Structural basis for ligand recognition of the neuropeptide Y Y2 receptor. Nat. Commun. 12, 737 (2021)
- Kaiser, A, Beck-Sickinger, A, Zhao, Q and Wu, B(2022). IP accumulation assay. Bio-protocol Preprint. bio-protocol.org/prep1809.
- Tang, T., Tan, Q., Han, S., Diemar, A., Löbner, K., Wang, H., Schüß, C., Behr, V., Mörl, K., Wang, M., Chu, X., Yi, C., Keller, M., Kofoed, J., Reedtz-Runge, S., Kaiser, A., Beck-Sickinger, A. G., Zhao, Q. and Wu, B.(2022). Receptor-specific recognition of NPY peptides revealed by structures of NPY receptors. Science Advances 8(18). DOI: 10.1126/sciadv.abm1232
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
