Advanced Search
Laser speckle perfusion measurements of brain
Last updated date: Dec 29, 2019 Views: 2349 Forks: 0
Title: Laser speckle perfusion measurements of brain.
Authors: Ramesh Chennupati, Kerstin Troidl, Stefan Offermanns
Abstract: Myogenic vasoconstriction is important for the regulation of organ perfusion. In this protocol we present the perfusion measurement method in brain using laser speckle contrast imaging.
Background: When laser light illuminates a diffuse object such as tissue, it results in a random interference pattern at the detector which is known as speckle pattern (Draijer et al., 2009). The movement of particles inside the tissue causes fluctuations in this speckle pattern resulting in blurring of speckle images when obtained with an exposure time equal to or longer than the speckle fluctuation time scale (Boas et al., 2010; Heeman et al., 2019; Milstein et al., 2016 ). This blurring can be related to blood flow if the fluctuations are caused by the movement of red blood cells (RBCs). This technique is widely used to measure the perfusion of burn wounds, retinal perfusion, cerebral blood flow (CBF) and skin microvasculature (Heeman et al., 2019).
Materials and methods: Isoflurane (Vetflurane, Virbac, Germany), Bupivacain (Actavis Deutschland GmbH, GermanyGermany), mineral oil (Sigma Aldrich, Germany), Bepanthen eye cream (Bayer, Germany)
Equipment:
MoorFLPI-2, Moor Instruments, UK
Stereotaxic frame (Stoelting, USA)
Software: moorFLPI Full-Field Laser Perfusion Imager Review Version 4.0
Procedure:
Cerebral blood flow measurements.
1. Place the mouse into the anaesthesia induction box containing 4-5% isoflurane.
2. After the mouse is unresponsive, remove it from the induction box. Confirm proper anesthetization by pinching toe. Apply eye ointment (artificial tears) on the eyes to prevent dryness.
3. Place the mouse in the prone position on a stereotaxic frame (pre heated to 37 °C) and connect it to a continuous flow of isoflurane.
4. Monitor the body temperature to ensure stable.
5. Before the skin is opened, local anesthesia is applied by infiltration of the area with Bupivacain (0,1 mg/kg). The skin is opened by a sagittal cut of around 1.5 cm along the midline. Carefully move the skin sidewards and fix it.
6. Fix the head in a stereotaxic frame.
7. Place mineral oil (2-3 drops) on the surface of the cranium to enhance the image quality by minimizing the effects of static scattering elements (Boas et al., 2010).
8. Change the concentration of isoflurane to 1.5 % and wait for 10 min.
9. After 10 minutes, live brain images were sampled at a sampling rate of 1 Hz for flux measurements.
10. A region of interest was defined (see photo and flux image), and the mean flow in that region was calculated and recorded in real time.
11. A mean image was calculated from the 60 perfusion images taken during a 5 minutes time span. Mean flux values were determined from the whole brain region. Fluxes in the ROIs were expressed as arbitrary units using a 256-color palette.
12. Animals are euthanized at the end of experiments under deep narcosis.
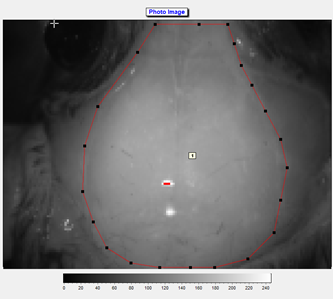
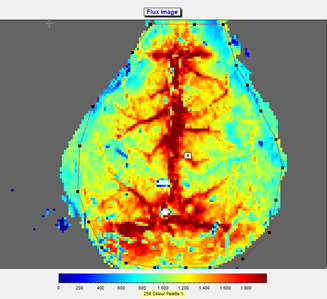
Notes:
1. Siblings or age and weight matching animals are used in the experiments.
2. The whole MoorFLPI-2 equipment was placed on an anti-vibration table.
3. The field of view and scanning resolution should be consistent between animals and all the measurements at different time points.
4. Avoid direct exposure of natural light during the procedure.
4. A 775-nm laser is used to illuminate the tissue (brain) and laser speckle images are acquired using a 568 × 760-pixel grayscale charge-coupled device camera at a sampling rate of 1 Hz. A 5 × 5 pixel window is used to calculate speckle contrast, and the maximal image resolution is 5 µm/pixel.
5. MOOR-FLPI translates the speckle contrast value into a relative perfusion unit giving information about blood flow, related to velocity of red blood cells. Pseudo-color images with the levels of perfusion scaled from blue (low perfusion) to red (high perfusion) will be obtained.
Competing interests: The authors declare no conflicts of interest.
Ethics: All animal care and experimental procedures in this study are approved by the local authorities (Regierungspräsidium Karlsruhe and Darmstadt, Germany). The approved animal proposal number for experiments is B2-1166.
References:
1) Boas DA, Dunn AK. Laser speckle contrast imaging in biomedical optics. J Biomed Opt. 2010;15(1):011109.
2) Draijer M, Hondebrink E, van Leeuwen T, Steenbergen W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci (2009) 24: 639. Lasers Med Sci (2009) 24:639–651.
3) Heeman W, Steenbergen W, van Dam G, Boerma EC. Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt . Journal of Biomedical Optics, 01 Aug 2019, 24(8):1-11.
4)Milstein DM, Ince C, Gisbertz SS, Boateng KB, Geerts BF, Hollmann MW, van Berge Henegouwen MI, Veelo DP. Laser speckle contrast imaging identifies ischemic areas on gastric tube reconstructions following esophagectomy. Medicine (2016) 95:25.
- Offermanns, S(2019). Laser speckle perfusion measurements of brain. Bio-protocol Preprint. bio-protocol.org/prep170.
- Chennupati, R., Wirth, A., Favre, J., Li, R., Bonnavion, R., Jin, Y., Wietelmann, A., Schweda, F., Wettschureck, N., Henrion, D. and Offermanns, S.(2019). Myogenic vasoconstriction requires G12/G13 and LARG to maintain local and systemic vascular resistance. eLife. DOI: 10.7554/eLife.49374
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
