Advanced Search
Phenotypic analysis of embryos
Last updated date: Jan 12, 2022 Views: 722 Forks: 0
Phenotypic analysis of embryos
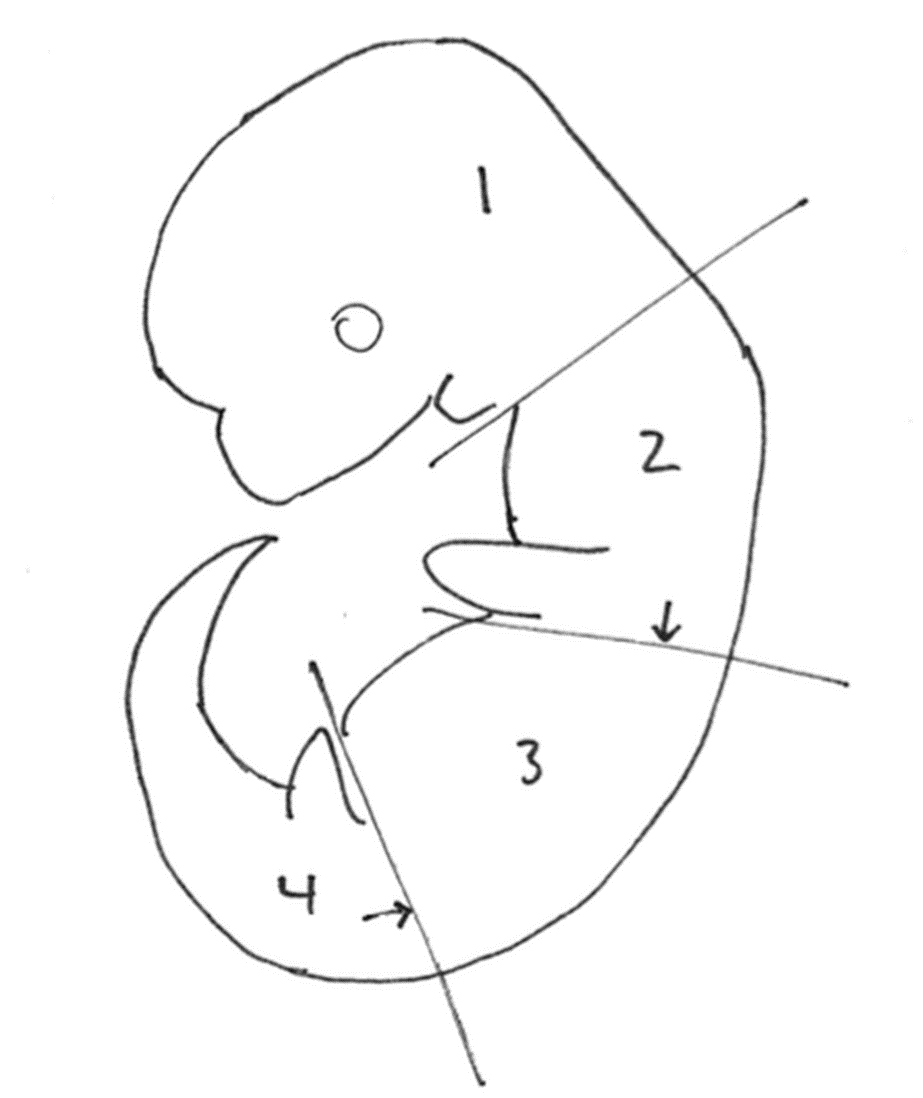
Embryos were dissected in cold phosphate-buffered saline (PBS). Each embryo was placed separately in the well of a 12-well plate and submerged in ~1 mL of 4% paraformaldehyde for a minimum of 1 hour at 4°C. After fixation, embryos were rinsed with PBS to remove excess PFA and replaced with a 30% sucrose solution in 0.1M phosphate buffer. Embryos were embedded separately in OCT (Tissue-Tek, Sakura). To isolate the specific regions of neural tube at the forelimb and hindlimb levels, embryos were separated into four pieces before embedding: the head was removed at the level of the branchial arches, then embryos were cut caudal to the forelimbs, and rostral to the hindlimbs (see Diagram). The second and fourth pieces were embedded with the limb bud-side down in the block, enabling the desired tissue to be collected immediately upon sectioning. Sections acquired were ~10 mm thick and placed on Superfrost Plus glass slides (Fisher). Slides were dried at least 30 minutes or overnight at 4°C.
Prior to antibody staining, sections were outlined with a hydrophobic pen and allowed to dry for 10-15 minutes or while the slides continued to dry after sectioning. Slides were then moved to a dark humidified chamber and rehydrated with 1X PBS for 10 minutes. The PBS was then replaced with 150-200 mL of antibody wash buffer (5% heat-inactivated goat serum, 0.1% Triton-X in PBS) and incubated for 1 hour at room temperature. After 1-hour, slides were incubated with 150-200 mL primary antibody in antibody wash buffer. Primary antibody was left on the slides overnight at 4°C in the dark humidified chamber. The following day, slides were rinsed with PBS or antibody wash buffer, three washes for 10-30 minutes each. Slides were then incubated in the dark with secondary antibody for 1-2 hours. After another three washes, ProLong Gold (ThermoFisher) antifade mountant was used to apply glass coverslips, which were then sealed with nail polish and allowed to cure overnight at room temperature in the dark before image acquisition. All solution changes were performed by careful aspiration from the long edge of a slightly tilted slide.
Recipes
Phosphate Buffered Saline, 10x [1.37M NaCl, 0.027M KCl, 0.1M Na2HPO4, 0.02M KH2PO4]. Combine 80g NaCl (Fisher BP358), 2g KCl (Fisher BP366), 14.4g Na2HPO4 (Fisher S374), and 2.4g KH2PO4 (Fisher BP362) in final volume of 1L. Solution can be sterilized by autoclaving or by filter sterilization. Dilute to 1x with dH2O.
4% PFA, dissolve 40g paraformaldehyde (Sigma P6148) in 700ml dH2O. Heat and stir in fume hood; use pH indicator paper strips to pH to ~7 using NaOH. When properly heated and at the correct pH, the solution should clear. Turn off the heat and add 100ml 10x PBS. Bring to final volume of 1L with dH2O. Filter through 0.45 micron filter, aliquot and freeze.
0.1M Phosphate buffer, pH 7.3. 69mL of 0.2M monobasic stock + 231mL of 0.2M dibasic stock. Bring to final volume with 600mL of dH2O.
0.2M monobasic stock. 13.9g sodium phosphate monobasic monohydrate (Fisher BP330 or Sigma S9638) in final volume of 500mL dH2O.
0.2M dibasic stock. 53.65g sodium phosphate dibasic heptahydrate (Amrexco 0348) or 28.4g sodium phosphate dibasic anhydrous (Fisher S374) in final volume 1L dH2O.
30% sucrose solution, w/v in 0.1M phosphate buffer. Ex: place 15g sucrose (Fisher BP220) in 50ml conical tube and fill to 50ml with 0.1M phosphate buffer. Shake vigorously to dissolve. Store at 4 degrees.
Diagram
- Gigante, E and Caspary, T(2022). Phenotypic analysis of embryos. Bio-protocol Preprint. bio-protocol.org/prep1499.
- Gigante, E. D., Taylor, M. R., Ivanova, A. A., Kahn, R. A. and Caspary, T.(2020). ARL13B regulates Sonic hedgehog signaling from outside primary cilia. eLife. DOI: 10.7554/eLife.50434
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
