Advanced Search
Cell transformation assay
Last updated date: Apr 7, 2021 Views: 1004 Forks: 0
2-stage cell transformation assay
This is a detailed protocol for the cell transformation assay used in Stefanius et al. [1], a modified version of the original protocol from Sakai & Sato [2].
Notes before starting the experiment:
➢ It is important to use 10% Bovine Calf serum (CS) instead of Fetal Bovine Serum (FBS). Cells growing in FBS containing medium, and also cells in low concentration of CS, have been shown to undergo spontaneous transformations after reaching confluency [3].
➢ NIH/3T3 cells with passage number <4 should be used for the transformation assay.
➢ Exosome concentration used should be determined through dose studies according to MISEV 2018 guidelines [4].
➢ Each test chemical is dissolved in DMSO (vehicle). The concentration of vehicle was below 0.2%, which did not affect the induction of transformed foci. Exosomes were suspended in PBS.
➢ Reagents needed:
o 12-O-Tetradecanoylphorbol-13-acetate (TPA) (Cell Signaling Technology, 4174)
o Methylcholanthrene (MCA), (Sigma-Aldrich, 213942–100 MG)
o Dimethyl sulfoxide (DMSO), (Sigma-Aldrich, D2650−5 × 5 ML)
o NIH/3T3 (ATCC, RRID:CVCL_0594)
o NIH/3T3 cells are maintained in Dulbecco's modified Eagle's medium (DMEM) (Millipore Sigma) supplemented with 10% (v/v) Bovine Calf serum (CS, Gemini) and 1% antibiotics solution (Penicillin-Streptomycin, Millipore Sigma).
o Cell lines are cultured at 37°C in a humidified atmosphere of 5% CO2.
o Crystal Violet, (Sigma-Aldrich, C0075)
Cell transformation assay
1. Prepare and warm complete growth medium (Dulbecco's modified Eagle's medium (DMEM)) (Millipore Sigma) supplemented with 10% (v/v) Bovine Calf serum (CS, Gemini), and 1% antibiotics solution (Penicillin-Streptomycin, Millipore Sigma) in a 37°C water bath.
2. Thaw the frozen stock of NIH/3T3 cells, spin down, aspirate the freezing medium and resuspend into 5mL fresh complete growth medium.
3. Plate in a 75cm3 cell culture flask, final volume 15mL.
4. Let attach and grow until ~70% confluency.
• Don't let cells grow confluent!
5. Trypsinize and replate actively growing cells with passage number <4 for the transformation assay at a density of 2.5 × 103 cells/well in a 6-well plate with 2 mL of culture medium.
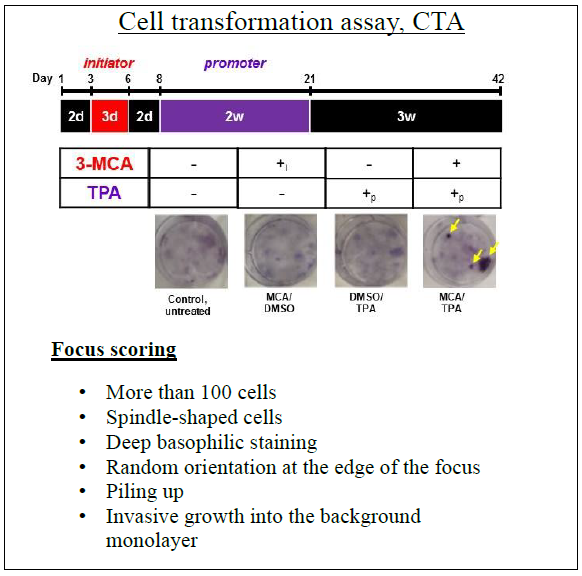
6. Two days after seeding, replace with media containing an initiator, either MCA (0.5 μg/mL), exosomes (determine appropriate concentration through dose studies [1, 4]), or 0.02% DMSO (control), and let cells grow for 3 days.
7. After 3 days replace the medium with fresh complete medium and let cells grow for an additional 2 days.
8. After 2 days, start promoter treatments with a medium containing, either TPA (300 ng/mL), exosomes (determine appropriate concentration through dose studies [1, 4]), or 0.2% DMSO (control), for 2 weeks. Change fresh medium containing promoter for three times a week (e.g. Mon-Wed-Fri).
9. Culture the cells subsequently in complete medium for 3 additional weeks replacing the medium twice a week (e.g. Tue-Fri).
Crystal Violet staining
10. Place 6-wells on ice and wash 2x with cold PBS (keep in refrigerator).
11. Fix cells by adding 1mL of ice-cold 100% methanol (store in freezer) for 10 minutes.
12. Move the cells off ice to room temperature. Aspirate methanol from plates and cover cells with 0.5% crystal violet solution in 25% methanol. Incubate for 2-10 minutes depending on how strong of stain is wanted.
13. Wash the cells in water several times, until the dye stops coming off. A fast way is to submerge the entire dish in a container full of cold running water, dump the water out from the dish and refill it a few times.
14. Let the 6-wells dry upside-down at room temperature at the bench overnight.
15. Analyze for focus scoring under the light microscope.
• Helpful tip, use Sasaki et al. for visual aid for the identification and the scoring of foci [5]. Below is shown a controlled experiment with yellow arrows pointing to foci.
References
1. Stefanius, K., et al., Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. eLife, 2019. 8: p. e40226.
2. Sakai, A. and M. Sato, Improvement of carcinogen identification in BALB/3T3 cell transformation by application of a 2-stage method. Mutat Res, 1989. 214(2): p. 285-96.
3. Xu, K. and H. Rubin, Cell transformation as aberrant differentiation: Environmentslly dependent spontaneous transformation of NIH 3T3 cells. Cell Research, 1990. 1: p. 197.
4. Théry, C., et al., Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 2018. 7(1): p. 1535750.
5. Sasaki, K., et al., Photo catalogue for the classification of foci in the BALB/c 3T3 cell transformation assay. Mutat Res, 2012. 744(1): p. 42-53.
- Stefanius, K and Orth, K(2021). Cell transformation assay. Bio-protocol Preprint. bio-protocol.org/prep1000.
- Stefanius, K., Servage, K., de Souza Santos, M., Gray, H. F., Toombs, J. E., Chimalapati, S., Kim, M. S., Malladi, V. S., Brekken, R. and Orth, K.(2019). Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. eLife. DOI: 10.7554/eLife.40226
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
