- Home
- Protocols
-
Tiny band for junction analysis after injection and no germline transmission

Dear,
I tried to create zebrafish reporter line based on this protocol two times, but both of them were not successful:
After injection, they showed tiny band for the junction fragments (controls-withount genomic sgRNA were no bands) - may I know if th
Read moreZexin Zhao Answered Mar 20, 2023
Hi Wesley,
Thank you for replying!
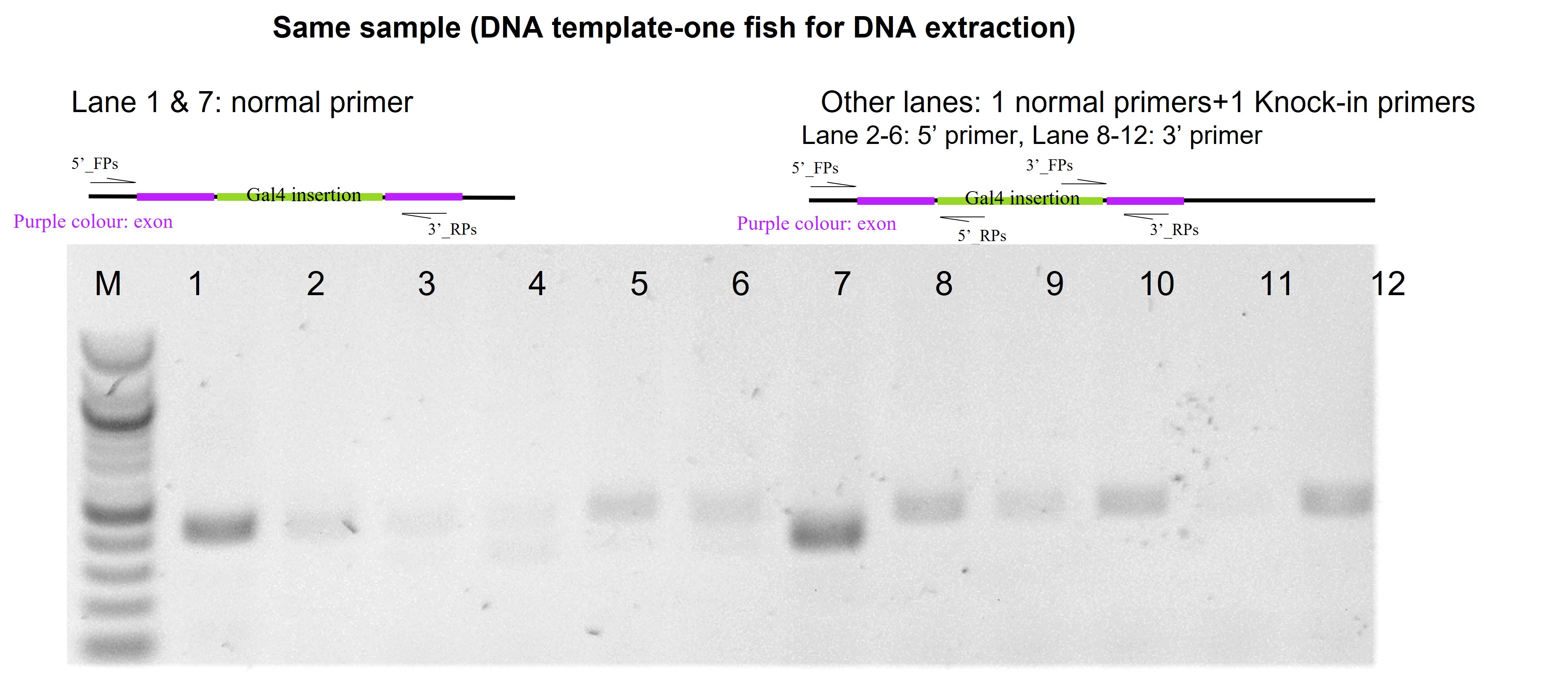
The insert is Gal4-VP16 (1757bp excluding 48bp homologous arm). And there was a visual selection (not 100% as fish was only allowed to be culture in the methylene blue, a little hard to do nursery selection).
For junction PCR (please have a look of the attached figure), the tiny bands are very light bands. I extracted DNA from one fish after injection and did PCR using either normal primers (both in the exon) or one normal primer+one knock-in primer, the former had a strong band but not the latter ones. Based on it, I think knock-in is very mosaic but I injected the CRISPR cassette in a very early stage, 5-10min after laying eggs. I'm not sure if this is the reason for no germline transmission (no bands for junction PCR and no fluorescent signals detected from F0 outcross).
Could you please give me some advice for optimization? Please let me know if anywhere that I have not described well.
Best wishes
Zexin
Wesley A. Wierson Author Answered Mar 20, 2023
LifEngine Animal Health Laboratories Incorporated
Hi Zhao,
What's the insert? What's the size? Is there a visual selection?
If you're seeing a "tiny" band (do you mean very light/not intense/not a strong band?) Can you add the gel?
Junction PCR is of course not quantitative but one can imagine the intensity of the band (ie. how much product you're getting) can reflect the amount of starting template. More engineered cells, more template, stronger band. It's possible there is just very mosaic knock-in and not efficient enough, early enough, to make it to germ cells.
Good luck!
Wes
My answer
Write your answer...
References (optional)
Add more
