Advanced Search
Adoptive cell transfer (ACT) and RNA-LPX treatment
Published: Dec 9, 2020 Views: 1029
Day of adoptive cell transfer (ACT)
Irradiate tumor bearing or non-tumor bearing WT mice 4 h prior ACT with sublethal dose of 2.5-5 Gy using an XRAD320 irradiator and a dose range of 0.44 Gy/min. No irradiation is needed for NSG mice.
ACT of murine T cells (alternative I):
Harvest murine transduced T cells from cell culture vessels into appropriate tubes.
Wash T cells with murine T cell medium once.
Layer carefully 5 volumes transduced T cells to 3 volumes Ficoll-Paque PREMIUM (1.084 g/mL).
Perform density gradient centrifugation with reduced acceleration and strongly reduced break for 20 min, 800 ×g to remove debris.
Harvest cells in lymphocyte layer and wash cells twice with PBS to remove remaining serum proteins.
Determine cell count of total viable cells.
Take 0.2-0.5 ×106 cells for transgene (CAR or GFP) expression analysis by flow cytometry.
Calculate the total amount of transgene (CAR or GFP) positive cells in total viable cells.
Adjust T cell numbers to transgene positive T cells/200 µL with PBS (e.g. 1 ×106 CAR expressing T cells/200 µL/ mouse).
Inject 200 µL of cell solution carefully into the retrobulbar venous plexus of anesthetized WT mice (e.g. by 2.5 % Isofluran inhalation anesthesia) with 30 G syringe (e.g. BD Micro-Fine).
ACT of human T cells (alternative II):
Harvest human transduced T cells directly from cell culture vessels into appropriate tubes after transduction process. Alternatively thaw frozen transduced T cells at 37°C and transfer cells into 10 volumes of human T cell medium supplemented with 2 ng/mL DNAseI and invert carefully.
Wash cells twice with PBS to remove remaining serum proteins.
Determine cell count of total viable cells.
Take approximately 0.2-0.5 ×106 cells for transgene (CAR surface) expression analysis by flow cytometry.
Calculate the total amount of transgene (CAR or GFP) positive cells in total viable cells.
Adjust T cell numbers to transgene positive T cells/200 µL with PBS (e.g. 1 ×106 CAR expressing T cells/ 200 µL/ mouse).
Inject 200 µL of cell solution carefully intravenously into the retrobulbar venous plexus of anesthetized NSG (e.g. by 2.5 % Isofluran inhalation anesthesia) with 30 G syringe.
Day of RNA-Lipoplex (RNA-LPX) treatment
Work under RNase-free conditions.
Bring the following stock solutions to room temperature: in vitro transcribed RNA (refer to RNA), nuclease-free water, 1.5 M sodium chloride and liposomes. F12 Liposomes contain 66.6 mol cationic DOTMA per 33.3 mol helper lipid DOPE in a ratio 2:1 – according to WO/2013/143555 and Kranz et al. 2016.
Calculate needed volumes of RNA, sodium chloride, liposomes and water for complexation. Thereby consider a charge ratio of 1.3 to 2 of cationic DOTMA and RNA for lipoplex mediated delivery into secondary lymphoid organs. The final concentration of sodium chloride should be 150 mM in generated RNA-LPXs.
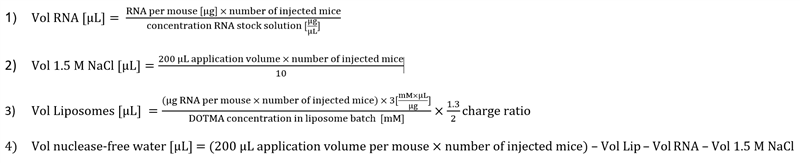
Mix thoroughly calculated volumes of nuclease-free water, 1.5 M sodium chloride, 20-40 µg RNA (encoding for e.g. tumor-antigen or T cell epitopes) in a nuclease-free tube. Add liposomes at last and vortex immediately for 10 seconds.
Incubate generated RNA-LPX 10 min at room temperature.
Inject RNA-LPX carefully intravenously into the retrobulbar venous plexus of anesthetized NSG (e.g. by 2.5 % Isofluran inhalation anesthesia) with 30 G syringe.
Solutions:
Murine T cell medium
RPMI1640-GlutaMAX supplemented with 10% (v/v) heat-inactivated FBS, 1x non-essetial amino acids, 1 mM sodium pyruvate, 10 mM HEPES, 50 μM β-Mercaptoethanol, 50 IU/mL Penicillin and 50 μg/mL Streptomycin.
Human T cell medium
X-VIVO 15 medium (Lonza) supplemented with 5% (v/v) human serum.
1.5 M NaCl solution
Dilute 1 mL sterile and RNase-free 5 M sodium chloride solution from e.g. Ambion with 2.33 mL nuclease-free water. Scale-up accordingly.
Reference
Kranz, Lena M.; Diken, Mustafa; Haas, Heinrich; Kreiter, Sebastian; Loquai, Carmen; Reuter, Kerstin C. et al. (2016): Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. In: Nature 534 (7607), S. 396–401. DOI: 10.1038/nature18300.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
