Advanced Search
Self-Paced Five-Choice Serial Reaction Time-Task for Mouse Behavioral Testing
*Contributed equally to this work Published: Apr 20, 2022 DOI: 10.21769/BioProtoc.4388 Views: 1244
Edited by: Arnau Busquets-Garcia Reviewed by: Nuria DaviuHSIU CHUN CHUANGRachael E. Hokenson
Abstract
The five-choice serial reaction time-task (5-CSRTT) is a behavioral rodent test that can assess executive functioning. It may be employed to analyze distinctive aspects of murine models for a plethora of diseases, such as attention deficit hyperactivity disorder, Alzheimer’s Disease, autism, and schizophrenia. The test has been performed primarily in rats and, although more challenging to apply to murine models, has the benefits of more diverse genetic models. The task is mainly based on training animals to nose-poke a lit screen within a stimulus duration time. Animals that perform a trial within the stimulus duration (SD) time receive a food/liquid reward. From training to experimental steps, classical 5-CSRTT can take more than 40 days to perform; however, here we present a murine self-paced, liquid reward-based 5-CSRTT (SP5C), using the ABET II system. Our version of SP5C can render competent results in ~14 days. We believe that, with this self-paced protocol, quicker results may be obtained using mouse models. Hence, a greater diversity of genetic models and diseases can be explored to expand our knowledge of complex polymorphic diseases, providing an additional platform for preclinical pharmaceutical testing.
Keywords: Five-choiceBackground
To experimentally evaluate impulsivity and novelty exploration is a challenging task, especially when considering murine models. The five-choice serial reaction time task (5-CSRTT) was conceived by Trevor Robbins, and first based on clinical assessments, such as the Continuous Performance Test, which is an adjunctive diagnostic tool in humans, as a manner to investigate aspects of executive functioning, such as attention deficit and impulsivity (Asinof and Paine, 2014; Cabral et al., 2020).
Thus, the 5-CSRTT can be used to analyze distinctive behavioral aspects of cognitive function, regarding processing speed, selective and sustained attention, impulse inhibition, and working memory (Bushnell and Strupp, 2009). These measurable phenotypes can widen our understanding of cognitive dysfunction observed in attention deficit hyperactivity disorder (ADHD), autism, schizophrenia, fragile X syndrome, Alzheimer’s, and Parkinson’s disease (Bushnell and Strupp, 2009; Asinof and Paine, 2014). Many of these disorders involve the frontal-subcortical-thalamic pathways, consisting of three main circuits: dorsolateral prefrontal (Klein et al., 2019), anterior cingulate, and orbitofrontal (OBF) circuits, which influence the organization of information, motivation, and emotional and limbic integration, respectively (Valera et al., 2005). 5-CSRTT has illustrated the effects of neurotransmitter modulation on executive function processes that utilize these complex circuits, such as mesolimbic dopamine’s influence on decisional speed and working memory, cortical acetylcholine’s influence on accuracy, forebrain serotonin’s effect on inhibition control, and ceruleocortical noradrenaline’s impact on selective attention (Bushnell and Strupp, 2009; Robbins, 2005). Thus, this flexible behavioral test expands the preclinical study of pharmacological treatments for complex neurocognitive disorders.
The 5-CSRTT behavioral task consists of presenting a light stimulus, shown within a 5-screen array, which remains illuminated for a set stimulus duration (SD), normally ranging between 0.8 and 16 seconds. After the stimulus duration has surpassed, the stimulus light turns off. The rodent can respond to the correctly lit screen either during the SD, or during the limited hold (LH) after the stimulus has turned off, which is typically 4 or 5 seconds (Posner et al., 2020). Upon a correct nose-poke, the animal receives a food or liquid reward at the reward area located across the experiment chamber. Once retrieved, the mouse must wait for the designated inter-trial interval (ITI) before beginning the next trial, which is initiated with the next stimulus illumination. Additionally, animals may be subjected to food or water deprivation, so they are compelled to perform the task (Note 1).
Several metrics are collected within an animal’s performed trials and are later used as phenotyping tools (Table 1 and Figure 1). The 5-CSRTT is typically performed in rats; mouse versions are available, but they are difficult to implement (Remmelink et al., 2017). Although rat behavior more closely mimics human behavior, mice are less expensive to maintain, and several genetically modified animal models are already readily available (Bryda, 2013). The traditional 5-CSRTT behavioral experimentation could lead up to 55 days when applied in mice (de Bruin et al., 2006; Oliver et al., 2009). We implemented a modified self-paced 5-choice (SP5C) method, which improves the efficiency of training to ~14–20 days. The ability to reduce training time is vital to mitigate animal handling, food and water deprivation, and even possible hormonal changes (Balcombe et al., 2004; Hurst and West, 2010). These factors can be potent stressors that affect experimental output. We have not observed any commercially available SP5C model that could steeply improve conditions of training, specifically for mouse models, motivating us to develop such self-paced method. The SP5C provides mice 12-hour access to the task, via a tunnel connecting the experiment chamber and their singly housed experiment home cage. After the training session, mice are returned to their original home cage with their littermates.
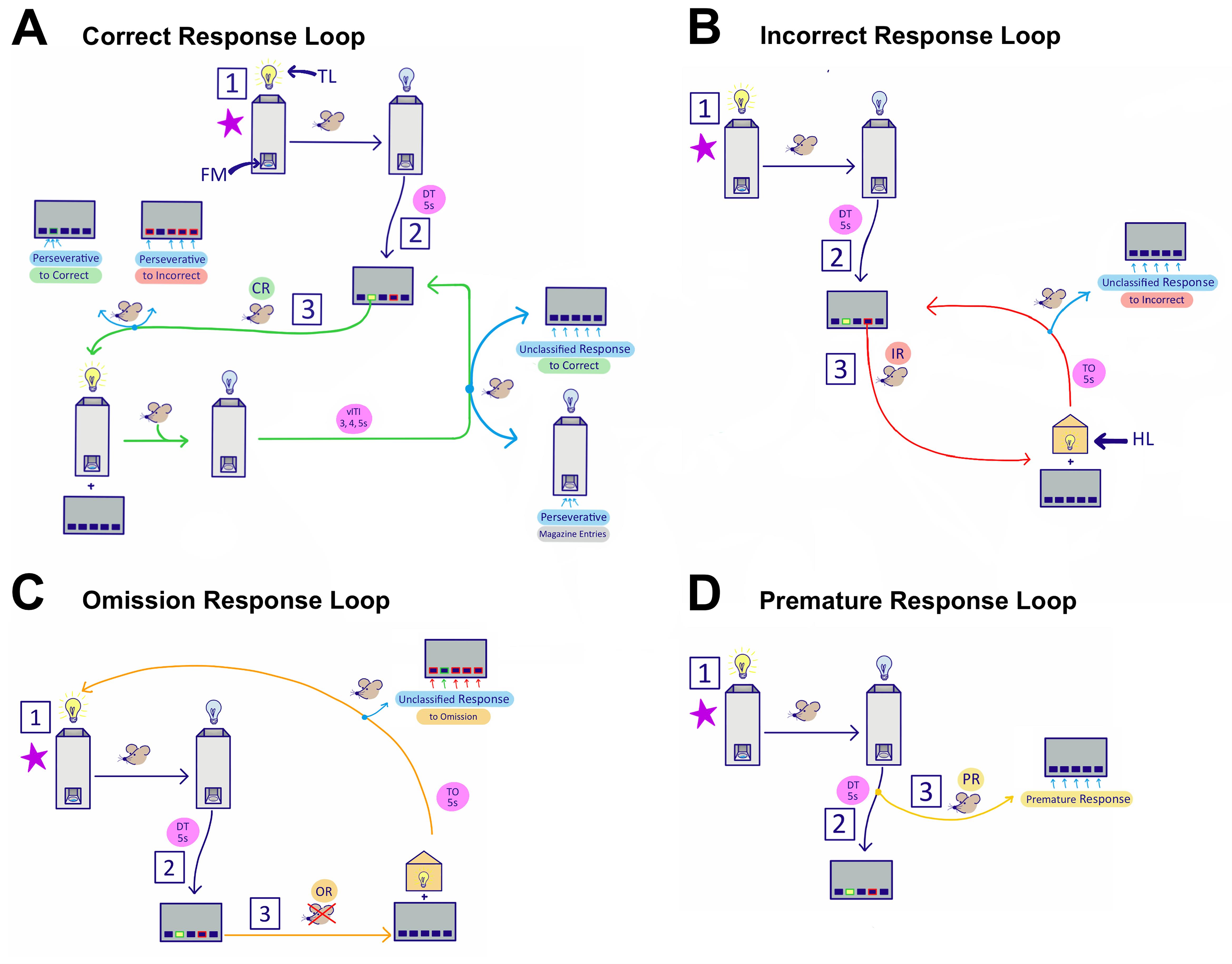
Figure 1. Self-Paced 5-Choice Method Flowchart and Metrics Obtained.
(1) The session begins with the tray light turned on and a reward delivery to the food magazine (FM). Once the mouse retrieves the reward from the tray, the tray light (TL) turns off, and the delay timer (DT, 5 s) initiates. (2) After the 5-s delay, one of the five stimulus lights pseudorandomly turns on for the set stimulus duration (SD). At this point, there are three possible responses: (A) correct response (CR), (B) incorrect response (IR), (C) omitted response (OR), or (D) premature response. (A) The CR loop is initiated when the mouse nose pokes the correct screen during the SD or after it turns off during the limited hold (LH, 4 s). The tray light turns on, and a reward is promptly delivered. Once the mouse retrieves the reward, the variable inter-trial interval (vITI, pseudorandom within 3, 4, or 5 s) timer begins, before the next stimulus is illuminated. Four other possible metrics are measured during the CR loop: Per-C, Per-I, Per-M, or Un-C. If the mouse continues to nose poke the screen before reward retrieval at either the correct screen (Per-C), or at the other four incorrect screens (Per-I), both count as a perseverative or compulsive behavior. During the vITI, after reward retrieval, continuous nose pokes to the screens are unclassified responses to correct (Un-C). Also shown are perseverative magazine entries (Per-M), which count continuous FM nose pokes during vITI, time out (TO), DT, SD, or LH. These entries are shown here within the CR loop for simplicity, but can occur during other loops. Please check for the full explanation of these metrics in Table 1. (B) The IR loop is initiated when the mouse nose pokes any of the four incorrect screens during the SD or LH. The screens turn off, and the house light (HL) promptly turns on for the duration of the TO (5 s), after which the next trial begins, and a stimulus is illuminated. Continued nose pokes to the screens during the TO count as unclassified responses to incorrect (Un-I). (C) The OR loop begins when the mouse does not nose poke any screen during the SD or LH. Then, the screens turn off, the HL turns on, and the TO timer begins. After the TO has passed, the TL turns on, and no reward is delivered. This signals as a “ready” light and allows mice to begin a trial when ready, thus accommodating for times when mice return to their experiment home cage to eat food or rest, without resulting in a series of false-positive ORs. Once the mouse is ready to begin the subsequent trial, it nose pokes the tray and responds to the illuminated stimulus after the 5 s DT. (D) A response made during the DT is counted as a premature response (PR). Please refer to Video 1 to see an example of mice performing each response loop in real-time.
Table 1. Variables that can usually be obtained during a 5-CSRTT experiment.
*Abbreviations: ITI: intertrial interval; TO: time out; DT: delay timer; SD: stimulus duration; LH: limited hold; CRL: correct response latency; DA: dopamine; 5-HT: 5-hydroxytryptamine; NA noradrenaline; GABA: γ-aminobutyric acid; GLUT: glutamate; NMDAR: N-methyl-D-aspartate receptor.
Due to the task’s complexity, rodents learn through incremental training stages to reach the “baseline stimulus duration,” whose value varies between studies. In our experience with C57BL/6, a baseline SD of 2 s was optimal; however, as outlined by Bari and colleagues, SD can extend to as low as 0.5 s (Bari et al., 2008). The phases include the preliminary training and the 5-CSRTT stimulus duration training phases. The preliminary training phase consists of three sub-stages: magazine training (MT), training stage 1 (T1), and training stage 2 (T2). The goal is to shape the rodent’s behavior, first to associate the location of the reward delivery (MT), then to associate the reward and task via response at any of the five illuminated screens (T1), and lastly to associate a correct response to a singly illuminated screen (T2). During this phase, the screens remain illuminated indefinitely (i.e., no SD) until the mouse completes a fixed number of trials (see Table 2 for full suggested criteria), and thus no omissions or premature responses are recorded. Following this preliminary phase, in the 5-CSRTT stimulus duration training phase the rodent must fulfill challenging criteria (Table 2) to progress from increasingly difficult, descending stimulus durations (SD 16, 8, 4, 2 s). Once the baseline SD is maintained at the required criteria for two consecutive sessions, the rodent is then subjected to the experimental sessions. This consists of six final sessions at baseline SD, in which averages of the final four-day measurements are used to compare phenotypes for the total trial count, accuracy, omissions, and premature responses. Modifications of the experimental portion, including variations in SD, ITI, pseudorandom auditory distractions, and alterations in stimulus brightness, can highlight and support phenotypic behavioral differences, as well as how they correspond with neurochemical modulations, as previously mentioned. The 5-CSRTT method provides a plethora of information to support the study of various disorders of cognitive function regarding attention, inhibition, and working memory. Here, we present an elaborate, self-paced 5-CSRTT protocol to be applied in mice, rendering fast and reproducible results.
Table 2. Summary of criteria for 5-CSRTT training progression. Advancement through training phases is based on completion of a fixed number of trials (MT, T1, T2, and SD) and/or when the animal achieves a pre-fixed level in determined variables (e.g., accuracy, omissions).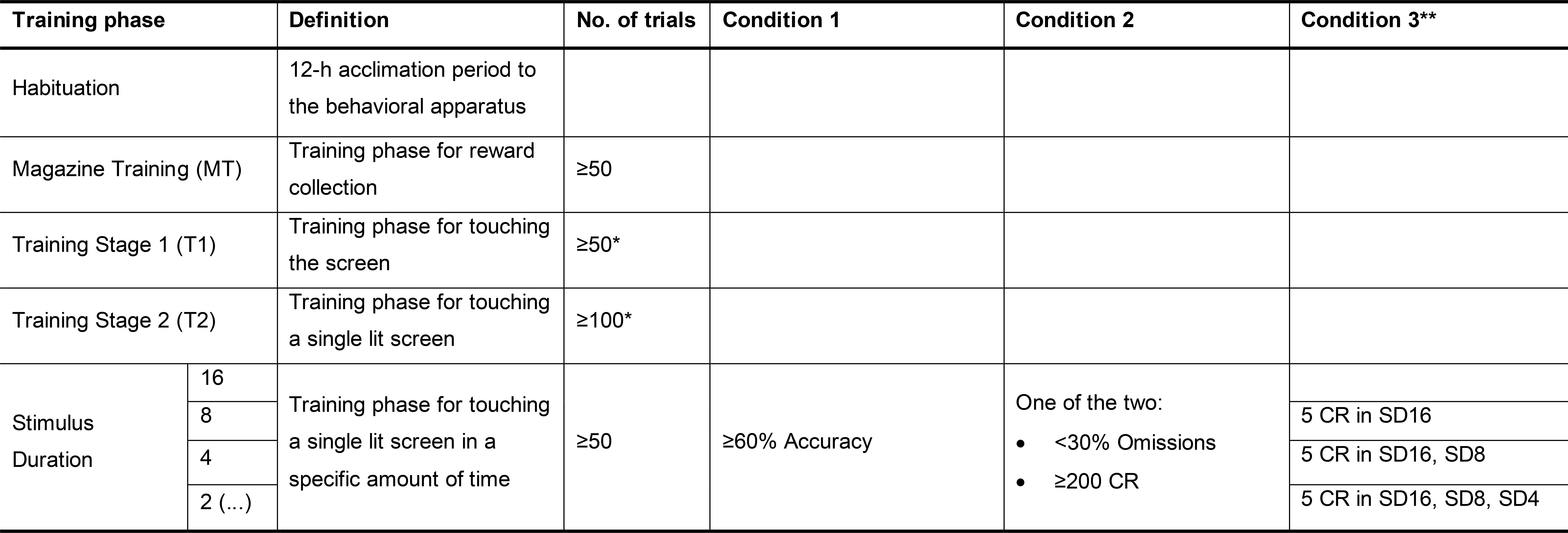
* The number of trials should be equal to the number of correct responses (CR).
** Condition 3 depicts the redo stages for a designated SD. It functions as a “warm-up,” requiring 5 CR in each of the previously graduated SD stages. We found that implementing “warm-up” stages was vital to ensuring mice continued their pace from the previous session, especially as they reached increasingly difficult SD stages.
Materials and Reagents
0.5 mL Insulin Syringe, 0.36 × 12.7 mm (Becton Dickinson, catalog number: 0216765)
50 mL Beaker (for storing Liquid Reward, Cole-Parmer, Vernon Hills, IL, USA)
Foam Board, 20” × 30” × ¼” (R. L. Adams Plastics Inc., Wyoming, MI, USA, Readi-Board). The foam boards are used to shut open spaces between the experiment chambers and the homemade shelf.
Black Trash Bags, 33” × 40” (Reynolds Consumer Products, Lake Forest, IL, USA). The trash bags are also used to shut the behavioral apparatus from environmental light.
PVC Pipes (Charlotte Pipe, Charlotte, NC, USA, Model Schedule 40 Truefit 4300 3” I.D., 3.5” O.D.) × 10”
Sucrose, Ultra-Pure, RNase-Free (Fisher Scientific, catalog number: ICN821713)
Citric Acid, Anhydrous, ≥99.5% Purity (Fisher Scientific, catalog number: BP339-500)
Sodium Chloride Solution, 0.9%, Suitable for Cell Culture (Sigma-Aldrich, catalog number: S8776-20 mL)
HydroPac® Water Pouch (Avidity Science, Waterford, WI, USA)
HydroPac® Sterile Disposable Valve (Avidity Science, catalog number: VX1300)
Chlorhexidine 2% Solution (VetOne, catalog number: 501027)
4% Sucrose Solution (see Recipes)
1% Citric Acid Solution (see Recipes)
Chlorhexidine Solution (see Recipes)
Equipment
5-choice Serial Reaction Time Task (5CSRTT), for Mice (Campden Instruments, Loughborough, UK, Bussey-Saksida Touch Systems, model: 89543)
Easy-Install System for Mouse Touch Screen Systems (Campden Instruments, Loughborough, UK, Bussey-Saksida Touch Systems, model: 80614-20)
Peristaltic Liquid Pump (Campden Instruments, Loughborough, UK, Bussey-Saksida Touch Systems, model: 80204)
Replacement Silicone Tubing, 0.5 mm bore × 1.6 mm wall × 3 m (Campden Instruments, Loughborough, UK, Bussey-Saksida Touch Systems, model: 80204-534)
Controller PC (Campden Instruments, Loughborough, UK, Bussey-Saksida Touch Systems, model: 88530)
Camera (Campden Instruments, Loughborough, UK, Bussey-Saksida Touch Systems, model: 80600-CAM)
Homemade shelf (as shown in Figure 2)
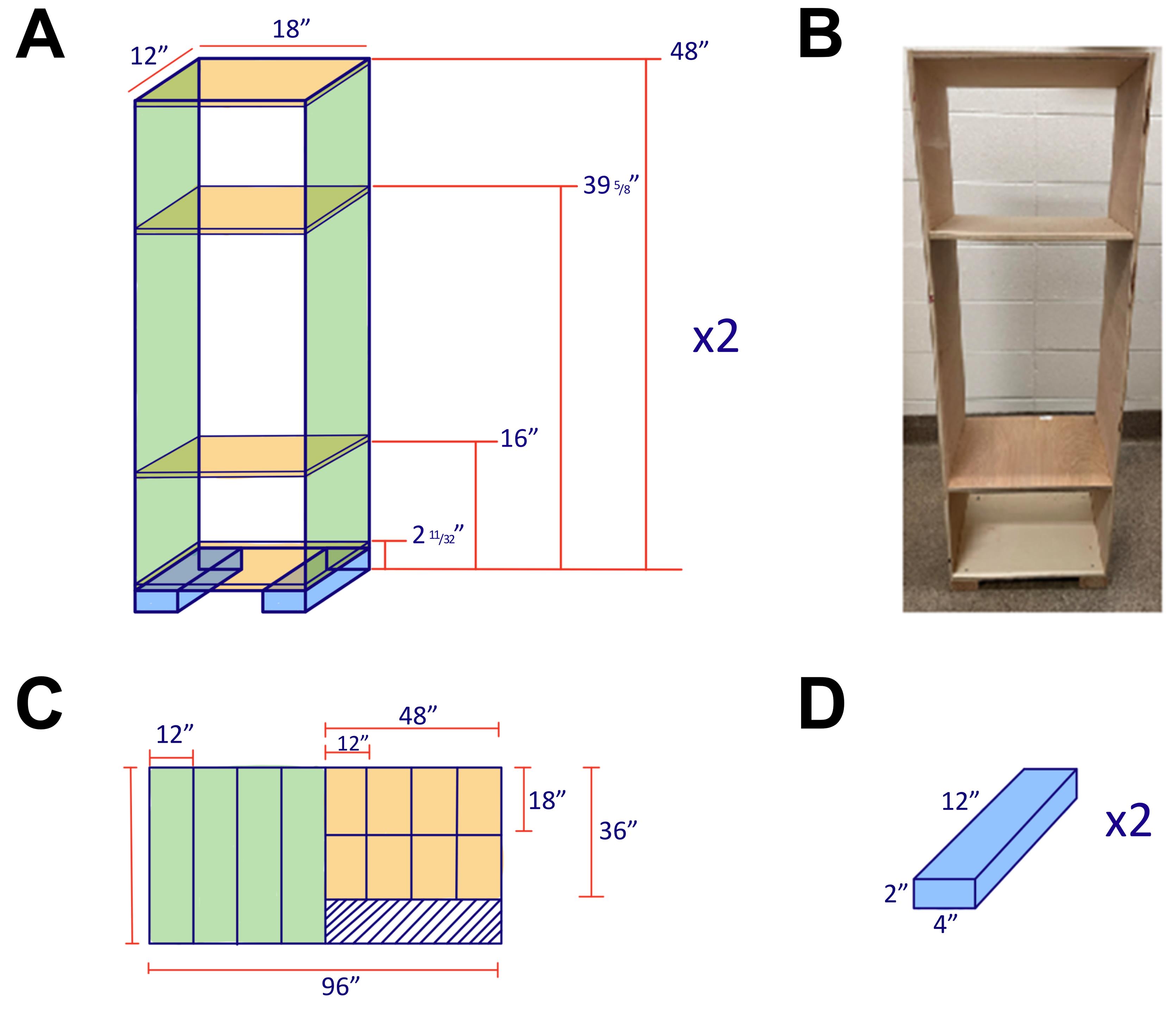
Figure 2. Diagram of Shelf attached to the 5CSRTT System.(A) The top shelf is 12” × 18” × 9”, the bottom shelf is 12” × 18” × 12”, and shelves are spaced are 23.63” apart. (B) A photo of the built shelf. (C) A 96” × 48” plywood is used to construct the individual shelves and side panels. (D) Two bottom panels attached under the shelves are 2” × 4” × 12”.
Cages 8.7” × 12.125” × 6.395” with 3” diameter hole (Thoren Caging Systems, Hazleton, PA, USA, model: #5)
Impulse Sealer (American International Electric, City of Industry, CA, USA, model: AIE-300)
Video Monitor (Samsung, Suwon, South Korea, model: S22A350H)
(Optional) Everbright Headlight (EverBrite, Hangzhou Greatstar Industrial Company, Huntersville, NC, USA, model: E021026A). This is a portable infrared headlight that can be used to assess experimental conditions in the dark and minimize mouse distress.
Software
ABET II Touch (Lafayette Instrument, Lafayette, IN, USA, https://lafayetteneuroscience.com/)
Whisker Multimedia (Lafayette Instrument, Lafayette, IN, USA, https://lafayetteneuroscience.com/). The WhiskerServer communicates registered touches on the screen to ABET II. It needs to be assessed daily to ensure the experiment chamber screens are functional.
QuadProcessor/Video Splitter (Lafayette Instrument, Lafayette, IN, USA, https://lafayetteneuroscience.com/)
Microsoft Excel 2016 (Microsoft, Redmond, WA, USA)
Prism (GraphPad, San Diego, CA, USA, https://www.graphpad.com/, Version 9.2.0)
RStudio (RStudio, Boston, MA, USA, https://www.rstudio.com/, Version 2021.09.0)
R (R Foundation for Statistical Computing, https://www.r-project.org/, Version 4.1.1)
Procedure
Category
Neuroscience > Nervous system disorders
Neuroscience > Behavioral neuroscience > Cognition
Medicine
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

