Advanced Search
Clearing of the Mouse Brain for Optical Imaging Using CUBIC
Published: Aug 20, 2021 DOI: 10.21769/BioProtoc.4123 Views: 1874
Edited by: Geoffrey C. Y. Lau Reviewed by: Xiaoyu LiuSubhra Prakash Hui
Abstract
Here, we describe a simple and efficient tissue clearing method, CUBIC (Clear, Unobstructed Brain/Body Imaging Cocktails and Computational analysis), which was first introduced in 2014 (Susaki et al., 2014). In the present study, the hemispheres of wild-type adult mice were cleared by standard procedures and were browning free with good transparency and no significant changes in tissue shape or volume over a short time frame. Further, in combination with immunofluorescence staining, 3D high-resolution images of tyrosine hydroxylase-positive dopaminergic neurons in the substantina striatum region were obtained by rotating-disc confocal microscopy, in addition to images of mouse cortical and hippocampal neurons. Considering the limitations of existing brain tissue removal methods, such as CLARITY, 3DISCO, and SeeDB, CUBIC boasts simple operation, high fluorescent protein preservation, and good antibody penetration. Moreover, CUBIC variants and optimizations are available for the 3D imaging of rodent, large primate, and human tissues to meet researchers' specific requirements, which could make it a popular option among research labs. Our study demonstrates the use of the CUBIC protocol for understanding brain structure and function in model animals with neurological diseases.
Graphic abstract: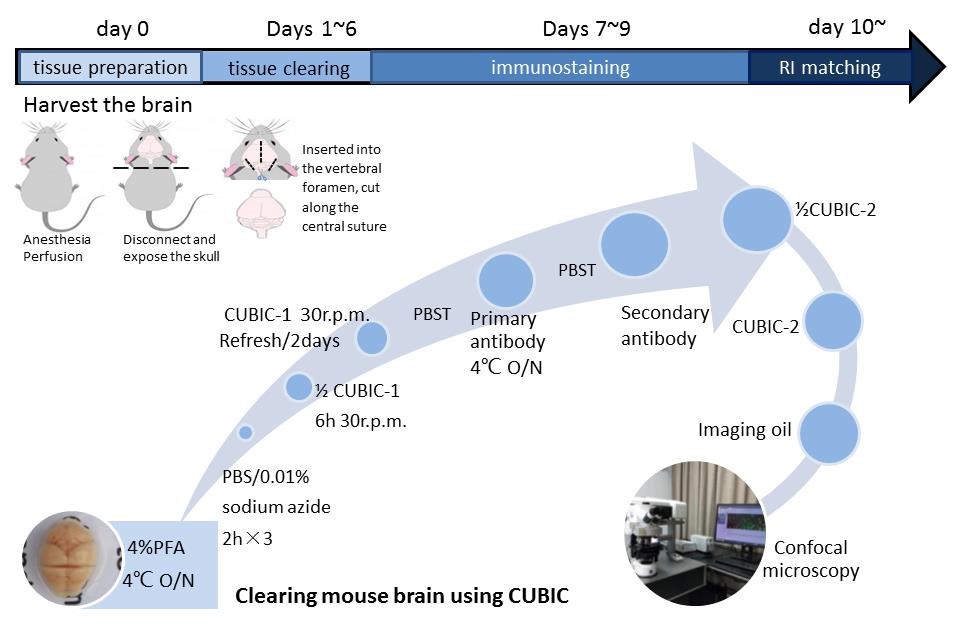
Flowchart of the CUBIC clearing and imaging protocol. A. Tissue preparation. The adult mouse was first perfused with cold PBS/PFA solution. The harvested brain tissue was fixed with 4% PFA on day 0. B. Tissue clearing. The tissue was incubated with 1/2-water-diluted CUBIC-1, resulting in the removal of hemochrome and lipid membranes. It usually takes 1-6 days to become more transparent. C. Immunostaining. Tissues (2-mm brain slice) were blocked and then stained with directly conjugated anti-TH diluent (1:100) and FITC diluent (1:500) on days 7-9. D. RI matching. Tissues were immersed in 1/2-PBS-diluted CUBIC-2 on day 10 to match the refractive index (RI). Following immersion in imaging oil for 2 h, the cellular position within tissues was photographed by confocal microscopy.
Background
Tissue optical clearing (TOC) enables the visualization of regions of interest in deep tissue and is now widely established. In recent years, a variety of methods have been developed based on organic solvents, water-based non-gels, and hydrogels (Ertürk et al., 2011; Chung and Deisseroth, 2013; Ke et al., 2013); however, both the CLARITY and 3DISCO protocols are associated with significant drawbacks: 3DISCO loses endogenous protein fluorescence and involves toxic and flammable substances (Matsumoto et al., 2019), while CLARITY relies on dedicated equipment for electrophoresis. In addition, acrylamide has carcinogenicity and toxicity in its aqueous form (Hogervorst et al., 2007). Compared with these other brain-clearing techniques, CUBIC is safe, simple to operate, and efficiently achieves transparency. This method clears fixed brain samples in a few days to render the brain transparent, thus meeting the quality requirements of optical imaging of the sample. Using the standard CUBIC tissue clearing procedure, we subject mouse brain samples to transcardial perfusion, clearing, immersion in antibody solution, and refractive index matching. The aminoalcohol used can effectively remove hemochrome from hemoglobin and myoglobin, which not only reduces light scattering but also lessens the absorption of light to achieve further decolorization and transparency. When combined with rotating-disc confocal microscopy, CUBIC facilitates comprehensive and quantitative analyses for understanding the brain structure on the millimeter-scale level. It boasts a simple clearance procedure (Muntifering et al., 2018), excellent clearance effect in a short time frame (approximately 11 days) (Wan et al., 2018), and outstanding fluorescence preservation (Tainaka et al., 2018), making it a popular option among research labs.
After performing the CUBIC protocol, the tissues are optically transparent (Richardson and Lichtman, 2015), which meets the quality requirements for the optical imaging of samples. In addition, it is compatible with traditional immunofluorescence methods (Xu et al., 2019) and can be used by researchers to explore the effects of immunolabeling. Combined with confocal, two-photon microscopy or light-sheet microscopy, CUBIC can easily obtain high-resolution (millimeter level) 3D brain images (Isogai et al., 2017; Irie et al., 2018) to provide information about the type, position, and activities of cells and cellular networks (Tomer et al., 2014), promoting research on the relationship between brain structure and function. CUBIC provides an integrative platform to advance scalable and collaborative 3D image analysis (Matsumoto et al., 2019) that enables a wide range of users to perform experiments targeting cellular and organ layers with multiple samples (Mano et al., 2020). This technology can solve developmental and fundamental neuroscience problems (Mano et al., 2018) and answer scientific questions associated with Alzheimer's disease (AD) (Liebmann et al., 2016) and other neurodegenerative diseases (Ueda et al., 2020).
Materials and Reagents
Blades (LEICA, catalog number: 819)
Microcentrifuge tubes, 2 ml (Biosharp, catalog number: 1810411)
Eppendorf tubes, 10 ml (Biosharp, catalog number: BS-100-M)
Intravenous (i.v.) injection needle, 21-G, butterfly type (GeneRulor, catalog number: SH12154)
Disposable syringes, 1 ml and 20 ml (NCS Testing Technology, catalog numbers: 20190629, 20191029)
Tinfoil (Heavy Duty, catalog number: 2345345)
24-well plates (Corning, catalog number: 3527)
Animals
Wild-type C57BL/6 (6-8 weeks of age) mice kept on a standard 12 h light/dark cycle; food and water were provided ad libitum.
Note: All experimental procedures and housing conditions were approved by the Animal Care and Use Committee of Guangdong Medical University.
Urea (Sangon Biotech, catalog number: A610148-0500)
Sucrose (Sangon Biotech, catalog number: A610498-0500)
N,N,N’,N’,-Tetrakis (2-hydroxypropyl) ethylenediamine (TCI, catalog number: T0781)
Triethanolamine (Sangon Biotech, catalog number: A690031)
Triton X-100 (Beyotime, catalog number: ST795)
PFA (MACKLIN, catalog number: 30525-89-4)
Tween 20 (Sigma-Aldrich, catalog number: P1379)
Sodium azide (Sigma-Aldrich, catalog number: ZY26628)
Heparin sodium (Tocris, catalog number: 2812/100)
HCl (Xilong Scientific, catalog number: 7647-01-0)
NaOH (GHTECH, catalog number: 1310-73-2)
Sodium azide (Nacalai Tesque, catalog number: 31208-82)
Anti-tyrosine hydroxylase antibody (Abcam, catalog number: ab112)
Goat anti-rabbit IgG H&L (FITC antibody) (Abcam, catalog number: ab6717)
Mineral oil (RI = 1.467; Sigma-Aldrich, catalog number: M8410)
Goat serum (Sigma-Aldrich, catalog number: G6767)
Phosphate-buffered saline (PBS) (1 M, pH 7.4) containing 0.1% Triton X-100 (PBST) (see Recipes)
4% (w/v) paraformaldehyde (PFA) solution (see Recipes)
Clearing solution (see Recipes)
CUBIC tissue removal reagent (see Recipes)
Equipment
Dissection tools required for standard mouse perfusion, e.g.,
Ophthalmic surgical scissors, 10 cm (straight) (Guangzhou Life Technology Co., catalog number: LG01-107-4H)
Standard tweezers (Guangzhou Life Technology Co., catalog number: LG01-105-3)
pH meter (Mettler Toledo, catalog number: FE28)
Magnetic stirrer (Tuhua, catalog number: 79-1)
Rocking bed hammock (Qilinbeier, catalog number: TS-3D)
2-8°C refrigerator (Haier, catalog number: SC-412)
Rodent Brain Matrices (TOW-INT TECH, catalog number: ST-2275)
Round-edged spoon (Guangzhou Life Technology Co., catalog number: LG01-111-2H)
Confocal microscope (Olympus, catalog number: FLUOVIEW FV3000)
Note: In this study, we used the FLUOVIEW FV3000 from Olympus for imaging objective lenses:
4× (NA = 0.16, WD = 13 mm)
10× (NA = 0.4, WD = 3.1 mm)
20× (NA = 0.7, WD = 1.8 mm)
Software
The following software is used as part of this bio-indentation protocol and data analysis process:
Microsoft Excel (Office 2016)
FIJI ImageJ (https://imagej.net/Fiji) (Version, 64-bit)
Procedure
Category
Neuroscience > Neuroanatomy and circuitry > Fluorescence imaging
Cell Biology > Tissue analysis > Tissue imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
