Advanced Search
Protocol for Spontaneous and Chaperonin-assisted in vitro Refolding of a Slow-folding Mutant of GFP, sGFP
*Contributed equally to this work Published: Vol 11, Iss 14, Jul 20, 2021 DOI: 10.21769/BioProtoc.4099 Views: 2907
Edited by: Emilia Krypotou Reviewed by: Ali Asghar Kermani
Abstract
Understanding the folding pathway of any protein is of utmost importance for deciphering the folding problems under adverse conditions. We can obtain important information about the folding pathway by monitoring the folding of any protein from its unfolded state. It is usually very difficult to monitor the folding process in real time as the process is generally very fast, and we need a suitable read out. In this protocol, we have solved this issue by using a protein that is non-fluorescent in its unfolded state but fluoresces in its native state after folding. The kinetics of refolding can be monitored by following the increase in fluorescence in real time. Previously, this was generally achieved by either monitoring a protein’s enzymatic activity or measuring the tryptophan fluorescence, where the signal output depends on well-described enzymatic activity or the frequency of tryptophan residues present in the proteins, respectively. Here, we describe a simple and real-time assay to monitor the refolding of sGFP, a recently described slow-folding mutant of yeGFP (yeast enhanced GFP). We unfold this protein using chemical denaturant and refold in a suitable buffer, monitoring the increase in fluorescence over time. GFP is fluorescent only when correctly folded; thus, using this technique, we can measure the true rate of protein refolding by following the increase in fluorescence over time. Therefore, sGFP can be used as an ideal model to study the in vitro protein folding process. Accordingly, the effects of different conditions and molecules on the protein folding pathway can be efficiently studied using sGFP as a model protein.
Graphical abstract: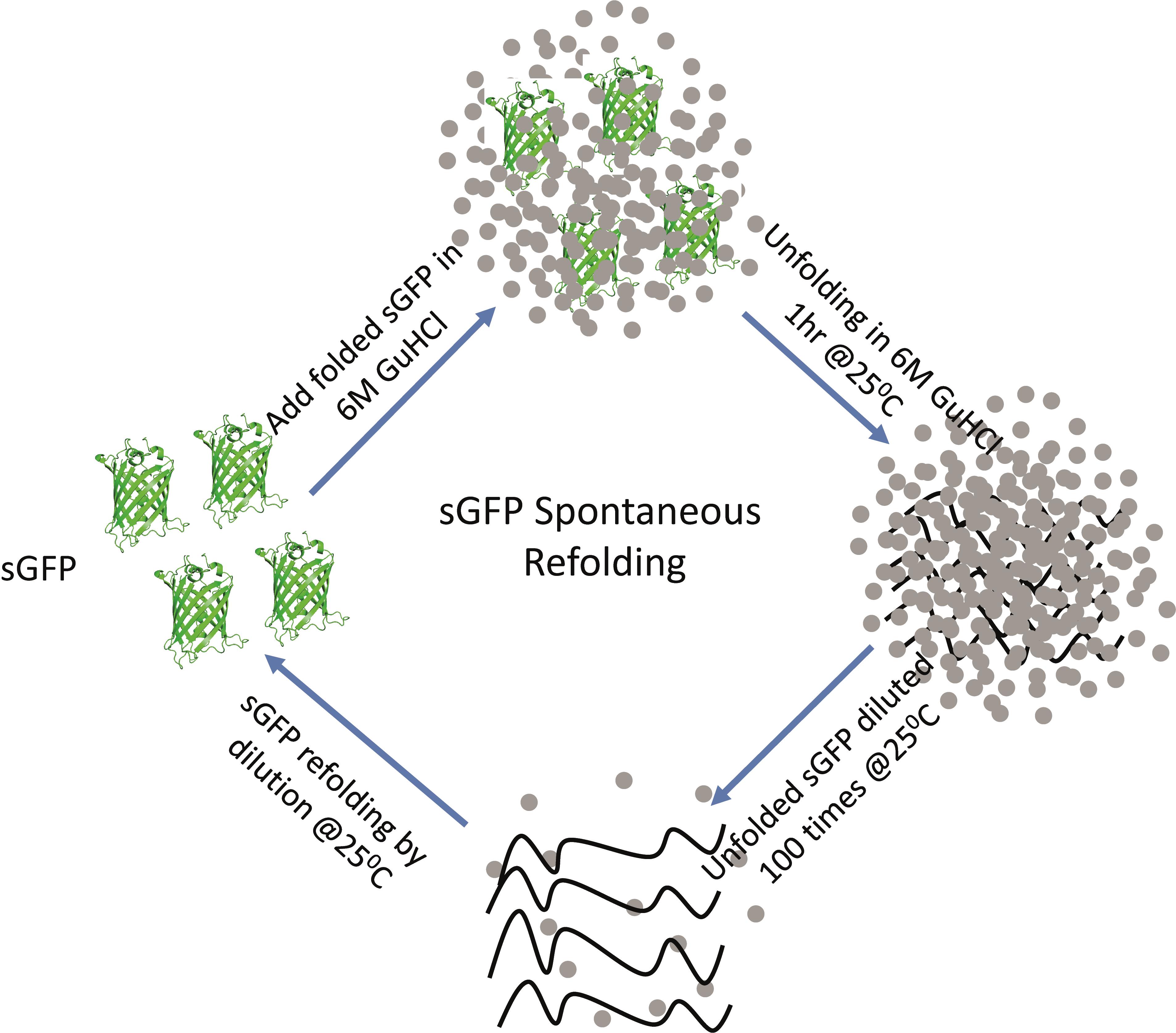
Schematic of the steps involved in the sGFP refolding pathway. Native sGFP is unfolded by chemical denaturation using 6 M GuHCl at 25°C for 1 hour and then refolded in refolding buffer by 100-fold dilution.
Background
Deciphering the folding pathway of proteins is one of the most explored topics in biology to date. Once synthesized, a nascent polypeptide chain is folded into its native structure, which is essential for its biological function. These polypeptide chains, especially the unfolded chains of small single-domain proteins, often fold spontaneously without any active assistance from other proteins. In contrast, larger multi-domain proteins frequently require folding assistance from proteins, commonly known as molecular chaperones, to fold into their native structure. The process of protein folding from nascent chains to correctly folded native states is extremely fast on a biologically relevant time scale. Thus, in order to study the steps of the folding pathway, we need to monitor the folding process using a slow-folding protein in a cell-free system, which is achieved by mimicking the cellular environment in vitro using suitable buffers. In an in vitro experiment, we unfold the test protein using a suitable denaturant and then refold it in a folding-favorable environment. Currently, the available protocols for measuring refolding rates are either based on measuring the enzymatic activity of proteins after they are refolded or monitoring the intrinsic tryptophan fluorescence of the protein (Weissman et al., 1994; Zahn et al., 1994; Makio et al., 1999; Doyle et al., 2003; Taniguchi et al., 2004; Kerner et al., 2005; Sharma et al., 2008; Malhotra et al., 2014; Jain et al., 2018). While measuring the refolding efficiency by estimating the enzymatic activity is one of the most suitable ways of checking the folded and active states of the protein, it largely depends on its well-known enzymatic activity. In the case of proteins without well-described enzymatic activity or activity that depends on the functional form of the protein, which may not form immediately after it is refolded, the estimation of refolding efficiency becomes difficult (Rospert et al., 1993). Another way of evaluating in vitro refolding is by measuring the tryptophan fluorescence, which again is largely dependent on the number of tryptophan residues and their location within the native structure (Chakraborty et al., 2010). We have devised an in vitro refolding assay of a recently identified in vivo substrate of the GroEL/ES chaperonin system (Sadat et al., 2020). In this protocol, we describe the methodology to monitor the refolding of a slow-folding yeGFP mutant protein, hereafter referred to as sGFP, in real time. We unfold the sGFP protein using guanidine hydrochloride as a chemical denaturant and subsequently refold the unfolded protein in a suitable refolding buffer in the presence and absence of chaperones (Sadat et al., 2020). The potential issues that are frequently encountered during in vitro refolding experiments are: (a) achieving complete unfolding of the protein in a reversible manner; (b) measuring the exact time taken by the unfolded protein to fold into its native form; and (c) accurately measuring the refolding rate, since most proteins fold very fast and this becomes a potential problem for measuring the in vitro refolding rate of a protein. Using sGFP successfully evades these potential problems in monitoring the refolding process in real time; therefore, our protocol can be a very useful guide for measuring the in vitro refolding rate of fluorescent proteins possessing similar characteristics to GFP.
Materials and Reagents
Tips: Sterile pipette tips (low-retention tips)
Falcon, 15-ml
Sterile microcentrifuge tubes (1.5-ml)
Wash bottle
Liquid discard beaker
Kimwipe tissue paper (Kimberly-Clark, catalog number: 34120)
Microcentrifuge tube rack
Falcon rack
Fluorescence cuvette (3.0 mm path length) (Hellma Analytics, catalog number: Z802336)
0.22-μm filter (Millex, catalog number: SLGP099RS)
10-ml syringe
MilliQ water (deionized water)
Methanol (100%) (HIMEDIA, catalog number: AS061-2.5L)
Guanidine hydrochloride (MP, catalog number: 105696)
Magnesium chloride (HIMEDIA MB, catalog number: 040-500G)
Potassium chloride (Sigma, catalog number: 793590)
Tris (ultra-pure) (MP, catalog number: 103133)
HCl (HMEDIA, catalog number: AS004-2.5L)
ATP (Sigma, catalog number: A2383)
DTT (MP, catalog number: 100597)
Purified protein (sGFP)
Purified chaperones and co-chaperones (GroEL, GroES)
sGFP (Sadat et al., 2020)
Buffer A (see Recipes)
Equipment
Pipettes: 2.5-μl, 10-μl, 200-μl, 1-ml
Centrifuge
Fluorescence spectrophotometer (Fluorolog 3 Spectrofluorometer, Jobin Yvon, Horiba)
Dry bath
pH meter
Procedure
Category
Biochemistry > Protein > Modification
Biochemistry > Protein > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

