Advanced Search
Efficient Assembly of Large Multiplex CRISPR/Cas9 Guide Arrays for Maize Genome Editing
Published: May 5, 2019 DOI: 10.21769/BioProtoc.3223 Views: 6181
Edited by: Marisa Rosa Reviewed by: Peter E Burby
Abstract
CRISPR/Cas9-based genome editing in maize is an effective tool, and researchers commonly wish to target multiple genes simultaneously. Transformation of maize is currently expensive and tedious, so researchers are incentivized to build vectors that target multiple genomic loci from a single transformation event. One way to accomplish this is to arrange Cas9 guides into a multiplex array that is transformed as a single locus to the plant (Char et al., 2017). These arrays can be long and repetitive, are challenging to build with traditional assembly methods such as restriction cloning, and are also difficult and expensive to synthesize. Golden Gate gene assembly (Vad-Nielsen et al., 2016) is a good answer to this challenge, as it is insensitive to the tandem repeats in these arrays. The MoClo system is an elaboration of Golden Gate cloning (Werner et al., 2012) and is particularly well suited for assembling larger multiplexed Cas9 guide arrays. In this protocol, we describe steps for designing and building a custom guide array targeting any number of maize loci using the MoClo standard components and syntax. We provide instructions for using variations of the maize and rice U6 promoters to drive guide RNA expression, but this system is generalizable for constructing guide arrays for other species as well.
Keywords: CRISPRBackground
Generating large arrays of guides is an efficient way to target multiple genomic loci from a single transformation event, which is especially relevant for species that are difficult to transform, like maize. These arrays have many small components and are repetitive, features that are difficult to achieve with traditional molecular assembly methods such as Gibson or restriction-ligation assembly, or newer gene synthesis methods. Golden Gate molecular assemblies utilize type IIS restriction endonucleases to guide the assembly of multiple DNA fragments into large constructs and is particularly well suited to building large Cas9 guide arrays. The MoClo system builds on Golden Gate cloning by utilizing a specialized syntax and set of vectors for the hierarchal iterative assembly of any number of DNA fragments in any arrangement (Werner et al., 2012). The MoClo system is therefore ideal for producing the large guide arrays that are an efficient way to edit multiple loci in difficult to transform systems such as maize.
Method overview: Here, we describe the process for Moclo assembly of a Cas9 guide gene array for gene editing in maize, using variants of maize and rice U6 promoters for driving guide RNA expression (Char et al., 2017; Qi et al., 2018). To accomplish this, the MoClo system is used in several steps to assemble small genetic parts into increasingly large and complex genetic constructs. In the Moclo language, three Level 0 parts are first produced: a promoter, a guide noticeable spacer, and an sgRNA scaffold. Next, these three Level 0 parts are assembled together to make a single Level 1 gene targeting a unique genomic locus. Multiple Level 1 genes with unique guide spacers are constructed in parallel, then assembled together to make a Level 2 multiplex array. Subsequent rounds of assembly can add more Level 1 genes to the array, with no theoretical limit to size. Finally, the array is transferred to an Agrobacterium expression vector to be used for maize transformation.
Materials and Reagents
- 1,000 μl, 200 μl, 20 μl, and 2 μl tips
- 1.7 ml Microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-129)
- Standard 90 mm Petri Dishes (Thermo Fisher Scientific, catalog number: 101VR20)
- Agrobacterium tumefaciens strain EHA101 (Life Science Market, catalog number: STR3010)
Note: Homemade preparations are also fine. - Chemically competent E. coli cells (One ShotTM TOP10 Chemically Competent E. coli) (Thermo Fisher Scientific, catalog number: C404010)
Note: Homemade preparations are also fine. Store for 6 months at -80 °C. - Maize B73 genomic DNA, diluted to 50 ng/μl
- Rice Nipponbare genomic DNA, diluted to 50 ng/μl
- Ultrapure water
- SOC media (Thermo Fisher Scientific, catalog number: 15544034)
Note: Homemade preparations are also fine. Store indefinitely at -20 °C. - The MoClo Toolkit (Addgene, catalog number: 1000000044) is highly recommended, although the components can be purchased individually
Note: This is a 96-well plate of frozen glycerol stocks. Store indefinitely at -80 °C. - Enzymes (generally last several years when stored at -20 °C)
- T4 DNA ligase (New England BioLabs, catalog number: M0202S)
- BsaI (BsaI-HF®v2) (New England BioLabs, catalog number: R3733S)
- BpiI (Thermo Fisher Scientific, catalog number: ER1011); Alternative BpiI Isoschizomer: BbsI-HF (New England BioLabs, catalog number: R3539S)
- DpnI (New England BioLabs, catalog number: R0176S)
- pENTRTM Directional TOPO® Cloning Kit, including pENTRTM cloning vector (Thermo Fisher Scientific, catalog number: K240020)
- Gateway cloning kit (GatewayTM LR ClonaseTM II Enzyme mix, Invitrogen, catalog number: 11791100)
- pMCG1005 Gateway cloning vector with Cas9 gene (lab stock)
- Standard LB bacteria growing plates with the appropriate antibiotics for cloning
- Antibiotics (will last several years when stored at -20 °C)
- Spectinomycin (Sigma-Aldrich, catalog number: S0692)
- Carbenicillin (Sigma-Aldrich, catalog number: C1613)
- Kanamycin (Sigma-Aldrich, catalog number: 60615)
- Rifampicin (Sigma-Aldrich, catalog number: R3501)
- High-fidelity DNA polymerase (Phusion® High-Fidelity DNA Polymerase) (New England BioLabs, catalog number: M0530S)
- Agarose I (Thermo Fisher Scientific, catalog number: 17850)
- Glycerol (Thermo Fisher Scientific, catalog number: 17904)
- Commercial DNA cleaning column kit (QIAquick PCR Purification Kit) (QIAGEN, catalog number: 28106)
- X-gal solution, ready-to-use (Thermo Fisher Scientific, catalog number: R0941)
- IPTG (Thermo Fisher Scientific, catalog number: 34060)
- Primers for amplification of promoters (Table 1)
Table 1. Primer sequence for amplification of promoters. All primers are listed in the 5’-3’ orientation, the Reverse primers are the reverse complement sequence of the 3’ end of the promoters. Both short and long versions of the U6 promoters can be used. The relative efficiency of each of these is unclear at this time. Different promoters are typically shuffled to drive guides. Note that entire Reverse primer is the reverse complement of the end of the promoter sequence, but that the four bases highlighted in orange will be used as overlaps in the Golden Gate assembly. Using these bases as the assembly overlap, rather than standard Moclo overlap sequences, is necessary to prevent assembly scars and ensure cellular transcription of guides begins at the intended nucleotide.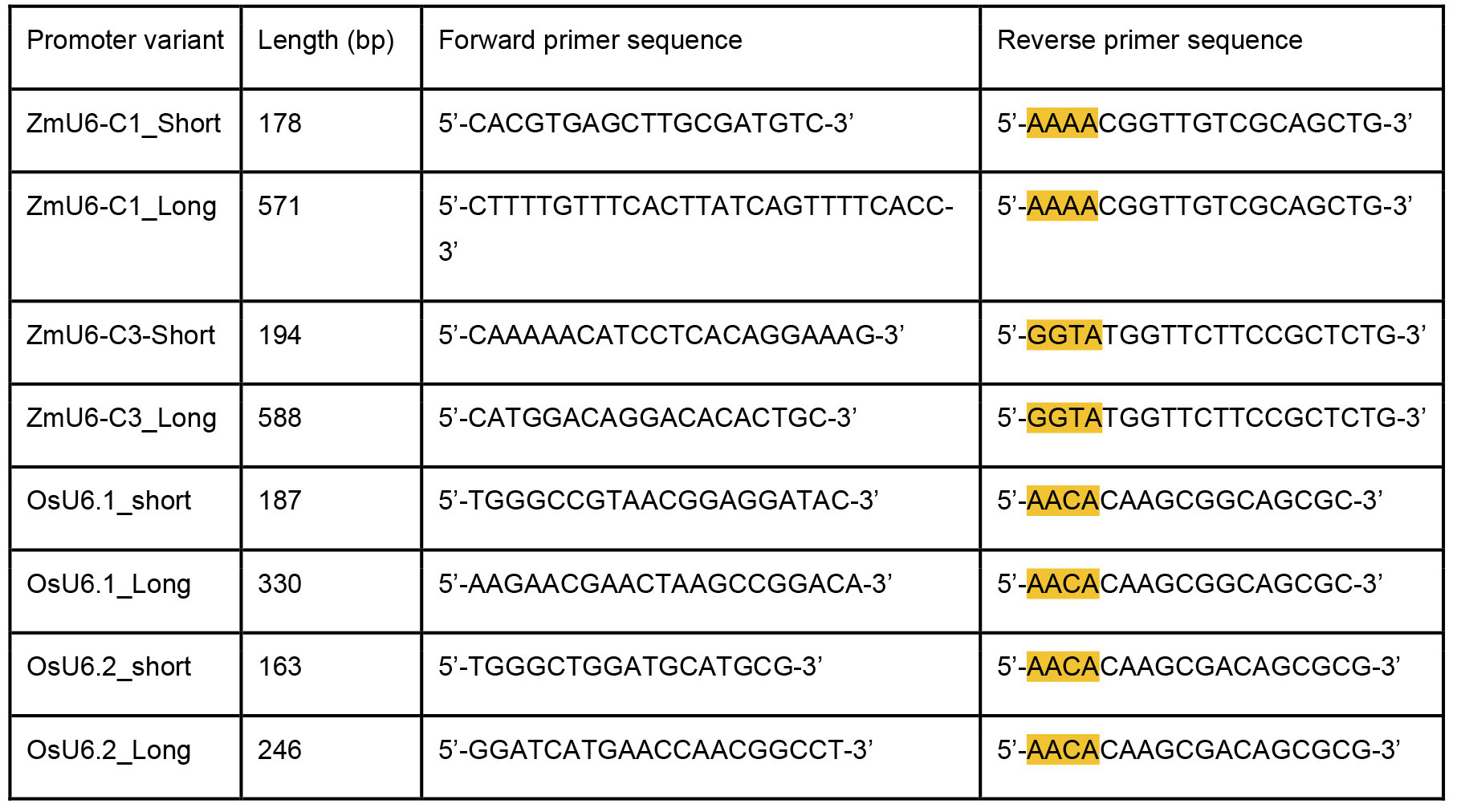
Equipment
- 1,000 μl, 200 μl, 20 μl, and 2 μl Pipettes
- Water bath (PrecisionTM General Purpose Baths) (Thermo Fisher Scientific, catalog number: TSGP02)
- Tabletop centrifuge (EppendorfTM 5424 Microcentrifuge) (Fisher Scientific, catalog number: 05-403-93)
- Spectrophotometer (NanoDropTM One) (Thermo Scientific, catalog number: 13-400-519)
- Thermocycler (EppendorfTM MastercyclerTM pro) (Fisher Scientific, catalog number: E950030010)
- Shaking incubator (Thermo Fisher Scientific, catalog number: 101VR20)
Software
- NEBioCalculator tool (http://nebiocalculator.neb.com)
- Genetic sequence analysis software such as Geneious (https://www.geneious.com) or Serial Cloner (http://serialbasics.free.fr/Serial_Cloner.html)
Procedure
Category
Plant Science > Plant transformation > Agrobacterium
Molecular Biology > DNA > DNA cloning
Molecular Biology > DNA > Transformation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

