Advanced Search
Isolation of human bone marrow non-hematopoietic cells for single-cell RNA sequencing
Published: Oct 19, 2023 Views: 762
Hongzhe Li1, Sandro Bräunig1, Stefan Scheding1,2
1Division of Molecular Hematology and Stem Cell Center, Lund University, Lund, Sweden;
2Department of Hematology, Skåne University Hospital, Lund, Sweden
*Corresponding author: Stefan Scheding, MD, Molecular Hematology, Lund Stem Cell Center, University of Lund, BMC B12, Klinikgatan 26, 22184 Lund, Sweden, Phone: +46-46-2223331, email: stefan.scheding@med.lu.se
Background
In the human bone marrow, hematopoietic stem cells (HSCs) and their progenies are contained in a specialized microenvironment regulating HSC maintenance and differentiation. Despite the important role of this hematopoietic environment (HME), its cellular composition, potential heterogeneity, and cellular hierarchy remain poorly defined. This is mainly due to the extremely low frequency of the HME-forming cells, the so-called bone marrow stromal (stem) cells, BMSCs [1].
The conventional approach to studying the human bone marrow microenvironment is mainly based on the analysis of different cell types by expression of a limited number of known surface markers, which results in an underestimation of cellular complexity. Novel single-cell-based omics approaches, on the other hand, have the potential to provide detailed insights into cellular organization and function. However, whereas bulk preparations of bone marrow cells allow for analysis of the majority of cells, important low-frequency cell populations such as BMSCs will escape detailed analysis.
Therefore, we developed a strategy to combine single-cell RNA sequencing of sorted non-hematopoietic BM cells with highly enriched BMSCs to resolve the cellular heterogeneity of the human bone marrow microenvironment at the highest possible resolution based on transcriptomic profiling [2].
Our approach is based on the expression of CD45, CD235a, and CD271. CD45 is a transmembrane protein tyrosine phosphatase encoded by the PTPRC gene (protein tyrosine phosphatase receptor type C). CD45 is considered a pan-hematopoietic marker and is widely used to select all hematopoietic cells and precursors except erythroid cells [3]. CD235a, also known as glycophorin A or GYPA, is a major intrinsic membrane protein of erythrocytes and a distinct marker of erythroblasts [4]. Therefore, we chose to use the combination of CD45 and CD235a to enrich human bone marrow microenvironment cells based on their low or absent expression of both CD45 and CD235a. Finally, BMSCs were highly enriched by sorting CD45low/-CD235a-/CD271+ cells, which is based on data by us and others demonstrating that the CD271 positive BM cell population contains all assayable stromal cells [5-7].
This paper describes a step-by-step protocol to isolate cells from the human bone marrow microenvironment for single-cell RNA sequencing [2].
Materials and Reagents:
Human iliac crest bone marrow aspirates from a healthy donor (ca. 50 – 60 ml), collected in 20 ml syringes prefilled with 1.6 ml Heparin (5000 IU/ml)
PBS w/o Ca2+ & Mg2+ (HyClone, catalog number: SH30256.01)
Gammanorm human normal immunoglobulins (Octapharma, catalog number: 096178)
Blocking buffer: PBS 1:50 Gammanorm, 1% FBS (sterile-filtered)
Ficoll-Paque Premium (Cytiva, catalog number: 17544203)
Collection buffer: PBS with 0.04% BSA
mouse anti-human CD45-FITC antibody (BD, catalog number: 345808)
mouse anti-human CD235a-PE-Cy5 antibody (BD, catalog number: 561776)
DAPI stock solution (1 mg/ml) (Sigma, catalog number: D9564)
mouse IgG1-FITC (BD, catalog number: 345815)
mouse IgG2b-PE-Cy5 (BD, catalog number: 555744)
mouse IgG1-APC (BD, catalog number: 345818)
Bovine Serum Albumin (BSA, Merck, catalog number: A7906)
Fetal Bovine Serum (FBS, Gibco, catalog number: 10270-106)
Ficoll buffer: PBS with 0.6% ACDA and 2% FBS
Sorting buffer: PBS with 1% BSA
mouse anti-human CD271-APC antibody (Miltenyi, catalog number:130-112-602)
Falcon conical tubes 50 ml
T-75 culture flask
Filcon, sterile, cup-type (BD, catalog number: 340626)
Equipment
- Pipettes (Eppendorf)
- Centrifuge (Hettich ROTANTA 460R)
Cell counter (NucleoCounter NC-250, chemometec)
BD FACS Aria II Cell Sorter
Software
BD FACS Diva Software
ChemoMetec NucleoView NC-250 (chemometec, version 1.2.0.0)
Procedures:
A. MNC isolation
- Prepare five 50 ml falcon tubes with 15 ml Ficoll-Paque Premium
- Transfer the bone marrow aspirate to a sterile T-75 culture flask
- Add 100 ml Ficoll buffer to the flask and mix
- Carefully layer 30 ml bone marrow-Ficoll buffer mix over Ficoll-Paque Premium in each 50 ml falcon tube
- Centrifuge at 300 × g for 30 minutes at room temperature without breaks [acceleration rate: 6 (maximum 10); deceleration rate: 0]
- Collect interphases into five new 50 ml falcon tubes and fill up with Ficoll buffer to 50 ml
- Centrifuge for 15 minutes at 400 × g at 4˚C
- Prepare 35 ml 1x Pharm Lyse by mixing 3.5 ml 10x Pharm Lyse with 31.5 ml sterile distilled water
- When centrifugation (step 7) is complete, aspirate the supernatants and resuspend the pellets by adding 7 ml 1x Pharm Lyse into each tube
- Combine samples from 5 tubes into one 50 ml falcon tube
Gently vortex the sample and keep it for 15 minutes at room temperature
Centrifuge at 200 × g for 15 minutes at 4˚C
- Carefully aspirate the supernatant
- Resuspend the pellet in 250 μl Blocking buffer
- Take 5 μl cells in blocking buffer and dilution to the concentration 5×104 to 5×106/ml
- Count cell number using NucleoCounter NC-250
- Adjust the cell concentration to ≤ 2×109/ml
B. FACS staining:
- Incubate cells in Blocking buffer for 20 minutes at room temperature
- **Aliquot the cells and add antibodies according to Table 1 to isolate CD45low/-CD235a- cells:
Table 1. FACS staining panel for CD45low/-CD235a- cell isolation
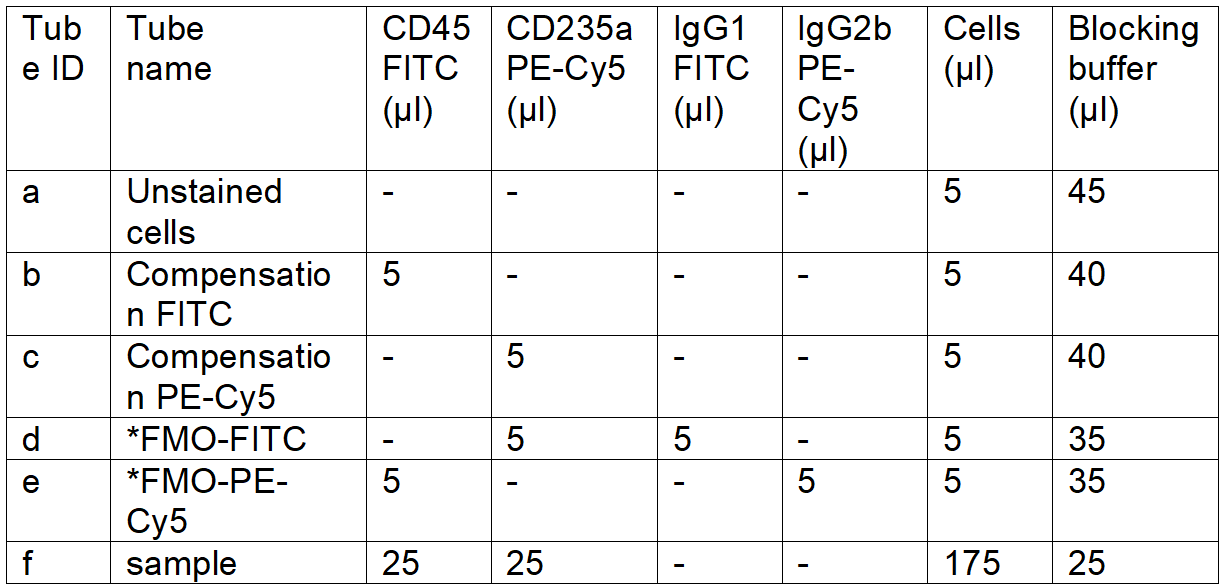
*FMO: fluorescence minus one control
2'. **Aliquot the cells and add the antibodies according to Table 2 to isolate CD45low/-CD235a-CD271+ cells:
Table 2. FACS staining panel for CD45low/-CD235a-CD271+ cell isolation
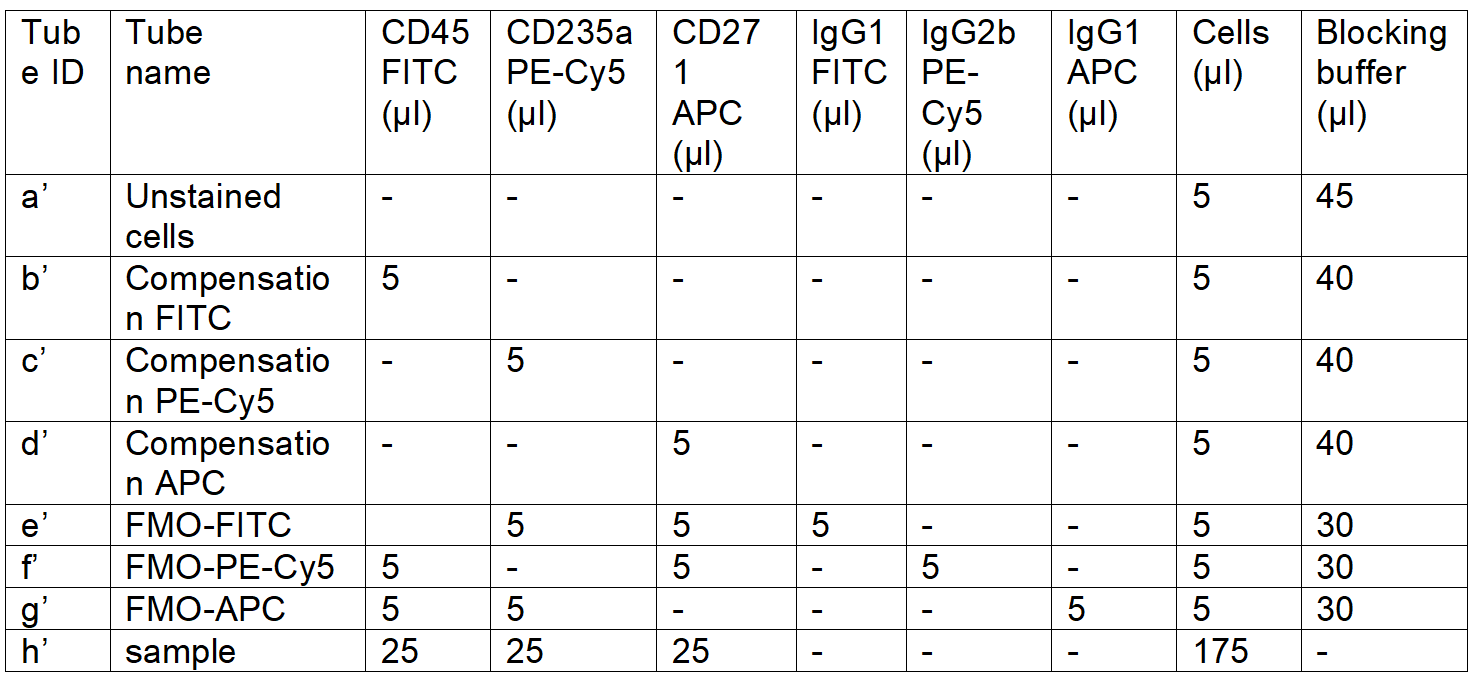
** Choose panel 1 for CD45low/-CD235a- cell isolation; choose panel 2 to enrich CD271+ cells
3. Incubate the staining tubes for 30 minutes at 4˚C in the dark
4. Wash the stained cells by adding 1 ml Sorting buffer to the tubes and centrifuge tubes for 5 minutes at 3000 rpm (800×g) at 4˚C
5. Resuspend Tube a-e (a’-g’ for panel 2) with 500 μl Sorting buffer and add 2.5 μl sterile DAPI stock
6. Resuspend Tube f (h’ for panel 2) in 3 ml Sorting buffer and add 15 μl DAPI stock
7. Pass the cells through a 30 μm Filcon or any equivalent strainer
8. Proceed quickly with FACS sorting.
C. FACS sorting
Create a Forward Scatter-Area (FSC-A) versus Side Scatter-Area (SSC-A) plot. Adjust the individual FSC and SSC photomultiplier tube settings to visualize the expected cell populations (Fig. 1A).
Set up a gating strategy to remove FSC-low populations, which consist of cell debris, air bubbles, and laser noise (Fig. 1A).
Create a Forward Scatter-Height (FSC-H) versus FSC-A plot and exclude doubles and multiplets by gating out cells with higher area signal values (Fig. 1B).
Create a FSC-A versus DAPI plot to exclude non-viable cells by gating out the DAPI-high cells (Fig. 1C)
Create a FITC versus PE-Cy5 plot to exclude CD45-high and CD235-expressing cells (Fig. 1D)
Create a FSC-A versus APC plot to exclude CD271 negative cells (Fig. 1E)
Sort 100 events from CD45low/-CD235a- or CD45low/-CD235a-CD271+ cell fraction into a tube containing 100 µl of ice-cold Collection buffer
Perform reanalysis with the sorted sample to evaluate the sorting purity (>85%)
Collect the target cell fraction into a tube containing 700 µl of the ice-cold Collection buffer
After sorting is complete, count the cell number and resuspend 20,000 cells with 47 µl of ice-cold Collection buffer
Proceed immediately to perform single-cell RNA sequencing
General notes
High-quality BM aspirates should be used freshly and contain sufficient numbers of cells. We routinely collect 50 – 60 ml bone marrow from 2 – 3 aspirations. However, lower volumes might also be sufficient. In case BM cells are obtained from biopsies, MNC isolation has to be performed by methods such as “crushing and/or flushing” either with or without the use of enzymes such as collagenase [8, 9].
CD45 is a transmembrane protein tyrosine phosphatase encoded by the PTPRC gene (protein tyrosine phosphatase, receptor type C). Conventionally, CD45 is considered a pan-hematopoietic marker and is widely used to select for hematopoietic cells. In this protocol, we chose to explore the human bone marrow microenvironment by using bone marrow mononuclear cells that showed low or absent expression of CD45. Our gating strategy aimed to enrich all non-hematopoietic cells but not to exclude potential stromal cells by too rigorous gating. Therefore, several hematopoietic cell types including B cells, NK cells, CD235a- late-stage erythroid progenitors, megakaryocytes, monocytes, dendritic cells, granulocytes, and CD34-expressing putative hematopoietic stem and progenitor cell (HSPC) population could be identified within this gate. This is consistent with previous murine studies which used comparable gating to enrich non-hematopoietic bone marrow cells and which included multiple CD45- hematopoietic cell populations [10, 11]. The hematopoietic cells could be easily identified based on their gene expression profiles.
Please note this paper describes the protocol to isolate all CD45-CD235a- cells from the human bone marrow microenvironment, which includes various cell types such as CD45-/low hematopoietic cells, endothelial cells, and stromal cells. It has been widely known that stromal cells are difficult to isolate due to the extremely low frequency of this cell type. We therefore recommend including CD271 in the staining panel (Table 2) to further enrich the stromal cells if this is the target population for detailed analysis as bone marrow stromal stem/progenitor cells are highly and exclusively enriched in CD271-expressing cells [5].
Having an effective Ficoll-Paque density gradient separation is one of the crucial steps in obtaining high-quality human bone marrow mononuclear cells for FACS-based cell isolation.
Bone marrow samples should be processed for mononuclear cell isolation immediately after aspiration (within 30 minutes) to achieve the best sample quality with the highest cell viability and cell yield.
It is important to use a swing-out rotor (also known as a bucket rotor) instead of a fixed-angle rotor for Ficoll-Paque gradient centrifugation to ensure a better separation of cell layers during centrifugation and a higher cell recovery rate. This is crucial when working with limited cell numbers or rare cell populations.
The duration of the red blood cell removal step (Procedure A11) can be adjusted depending on the individual sample. Pharm Lyse treatment can be reduced to as short as 2 minutes if red blood cell contamination is minimal.
Although we used CD45-FITC, CD235a-PE-Cy5, and CD271-APC antibodies in this protocol to distinguish CD45/CD235a double-negative cells from the cells that are positive for one or both of the surface markers and to enrich CD271-expressing cells, any fluorochromes conjugated to these three antibodies could be used to isolate cells from the human bone marrow microenvironment. When selecting antibodies with distinct conjugates, it is advisable to opt for conjugates that have minimal overlap in their emission spectra. As FACS-based cell isolation is highly dependent on the quality of antibody staining, it is recommended to titrate each antibody using bone marrow samples to avoid the antibody saturation effect, optimize signal- to- noise ratio, minimize antibody non-specific binding, enhance consistency and reproducibility, and achieve the best sorting outcome.
As the flow cytometric cell sorter plays an essential role in obtaining accurate and reliable results in this protocol, it is extremely important to ensure the cell sorter is operated under optimal conditions.
Machine-specific preparatory steps should be performed according to the manual.
The cell sorter should be cleaned properly before the experiment to minimize any potential contamination.
Photomultiplier tube (PMT) voltage and detector sensitivity settings need to be adjusted for each fluorochrome (FITC, PE-cy5, APC, and DAPI).
Perform compensation calculation using single-stained compensation controls. Double-confirm the compensation calculation by using FMO controls need to be included.
A gating strategy to distinguish positive and negative events based on FMO samples has to be established.
The sample flow rate has to be kept within the recommended range to avoid overlapping events (<3000 events/second for 70 µm nozzle, BD Aria II)
- Last, as human samples generally demonstrate high person-to-person diversity, it is recommended to collect as many samples as possible to ensure reproducible results.
In summary, we present a protocol for the isolation of human bone marrow microenvironment cells through a combination of Ficoll-Paque density gradient centrifugation, fluorescent antibody staining, and FACS sorting. In this protocol, we detail the critical steps for obtaining high-quality mononuclear cell fractions and emphasize the importance of antibody titration and proper instrument setup. This protocol provides a valuable resource for researchers aiming to isolate and characterize human bone marrow microenvironment cells with the highest precision and reliability, offering insights into the intricate cellular components of this critical niche for hematopoiesis and immune regulation.
- Pittenger, M.F., et al., Multilineage potential of adult human mesenchymal stem cells. Science, 1999. 284(5411): p. 143-7.
- Li, H., et al., Identification of phenotypically, functionally, and anatomically distinct stromal niche populations in human bone marrow based on single-cell RNA sequencing. Elife, 2023. 12.
- Penninger, J.M., et al., CD45: new jobs for an old acquaintance. Nat Immunol, 2001. 2(5): p. 389-96.
- Mao, B., et al., Early Development of Definitive Erythroblasts from Human Pluripotent Stem Cells Defined by Expression of Glycophorin A/CD235a, CD34, and CD36. Stem Cell Reports, 2016. 7(5): p. 869-883.
- Tormin, A., et al., CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood, 2011. 117(19): p. 5067-77.
- Jones, E.A., et al., Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum, 2002. 46(12): p. 3349-60.
- Quirici, N., et al., Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol, 2002. 30(7): p. 783-91.
- Ahrens, N., et al., Mesenchymal stem cell content of human vertebral bone marrow. Transplantation, 2004. 78(6): p. 925-9.
- Gleitz, H.F.E., et al., Isolation of human bone marrow stromal cells from bone marrow biopsies for single-cell RNA sequencing. STAR Protoc, 2021. 2(2): p. 100538.
- Baryawno, N., et al., A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell, 2019. 177(7): p. 1915-1932 e16.
- Boulais, P.E., et al., The Majority of CD45(-) Ter119(-) CD31(-) Bone Marrow Cell Fraction Is of Hematopoietic Origin and Contains Erythroid and Lymphoid Progenitors. Immunity, 2018. 49(4): p. 627-639 e6.
Figure legends
Figure 1. Gating strategies for isolation of human bone marrow CD45low/-CD235a- and CD45low/-CD235a-CD271+ cells. Representative FACS plots illustrate the sequential gating strategy to eliminate cell debris (A), exclude doublets (B), and dead cells (C). Sorting gates for isolation of CD45low/-CD235a- (D) and CD45low/-CD235a-CD271+ (E) cells according to the appropriate FMO controls.
Related files
 Fig1_bioprotocol.pdf
Fig1_bioprotocol.pdf Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
