Advanced Search
Cell cultures
Published: Jan 15, 2022 Views: 718
Primary microglia culture protocol
Prepare ahead of time
- Neurobasal-A (NBA) (10888022, Gibco) with 1% antibiotic/antimycotic (15240096, Gibco, abbreviated PSF)
- Culture medium: DMEM with 4.5 g/L glucose containing 1% PSF , 2 mM GlutaMAXTM (35050061, Gibco), 10% heat inactivated FBS* (10082147, Thermo Fisher Scientific), 50 ng/ml murine m-CSF** (315-02, Peprotech).***
- Digestion solution: 5 ml NBA/PSF + 6.2 mg papain (solid, ~21U/mg, ~70% protein, Worthington Biochemicals or 0.4 ml Papain slurry (P3125, Sigma 2×Crystallized, ≥16 units/mg protein) + 1.6 mg L-cysteine (C7352, Sigma). Warm to 37˚C to dissolve fully and wait until solution is clear.
- Light Inhibitor (LI) solution: 1 ml HI + 9 ml NBA/PSF
- Heavy Inhibitor (HI) solution: 5 ml (NBA/PSF) + 50 mg trypsin inhibitor (T9128, Sigma) + 50 mg BSA (B5702, Sigma).
- Dissociation solution: 5 ml NBA/PSF containing 25 µl DNaseI
- DNAse I (D5025, Sigma, 150 KU bovine pancreas) 2 mg/ml
- Filter through 0.2 µm pore filters to ensure solutions are sterile. Sterilise all tools by either dipping in 70% ethanol just before use or by autoclaving..
Dissection
- Decapitate 2-4 neonatal pups and submerge head in ice cold NBA/PSF
- Remove brain and peel meninges using forceps****
- Cut away the olfactory bulbs and cerebellum using a sterile razor blade
- Slice the brain into 3 coronal sections
- Blunt dissect away the cortex using forceps
- Place cortices in the digestion solution and cut into smaller chunks using fine scissors or a 1 ml pipette. Add DNase I, 50 µl/ml.
- Place cut cortices into 37˚C/5% CO2 incubator for 20 mins, swirling every
- Remove enzyme solution and add 1 ml LI solution
- After 1-2 min, remove LI and replace with 1 ml HI
- Move tissue chunks to 15 ml falcon tube containing the dissociation solution
- Using a fire polished glass pipette or a P1000 tip), very gently dissociate the chunks until a mostly homogenous mix is formed
- Allow to settle for 2 minutes*****
- Collect the supernatant that forms over a visible white pellet (chunky junk but retain until you are sure you have cells to plate),
- Spin supernatant for 4 mins at 1.4 x g
- Aspirate supernatant (retain until certain you have cells to plate) and resuspend the cell pellet in 1 ml culture medium.
- Plate the suspension onto non-TC treated 10 cm petri dishes (non-TC so only the microglia can stick down) in 10 ml culture medium.
- Check for presence of cells under phase contrast microscope
- Place in incubator. DO NOT CHECK OR TOUCH CELLS FOR 7 DAYS to facilitate attachment.
- Replenish media at 7 DIV and then every 3 days.
- To split cells, wash once with PBS (minus Ca and Mg) and scrape carefully in circular motion from edge to centre of the plate. Collect, spin, and resuspend gently in culture medium. Cells can be replated and expanded on tissue culture plastic. They will adhere to glass coverslips without coating.
Notes
* We have also successfully used FBS from this source (F9665, Sigma). You can also heat inactivate FBS in a 60% water bath for 30 minutes. Cool to room temperature and spin to remove any insoluble material.
** Recombinant murine mCSF can be replaced with conditioned medium from L929 cells collected from a confluent culture in the same DMEM medium and frozen at -20˚C in aliquotes. Dilute 1:5 into the culture medium.
*** This enriched DMEM also works: 10569-010, Gibco
**** Meninges are more difficult to remove when pups are older but it is important to remove them as the meningeal cells with also grow and contaminate the culture
***** The “pellet” can vary in size but even if it appears quite substantial it should be left undisturbed; there are plenty of microglia in the supernatant.
Images
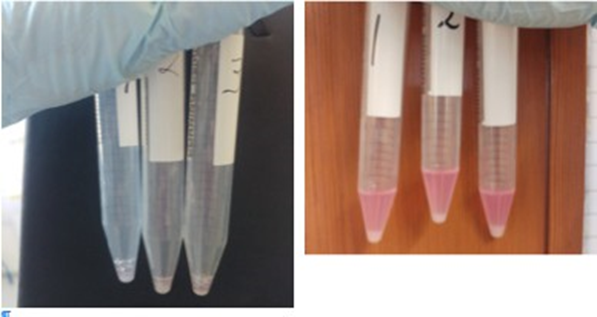
Left panel, remaining loose tissue after dissociation, supernatant removed Right panel, Pellet of cells after spinning 4 minutes at 400 rpm
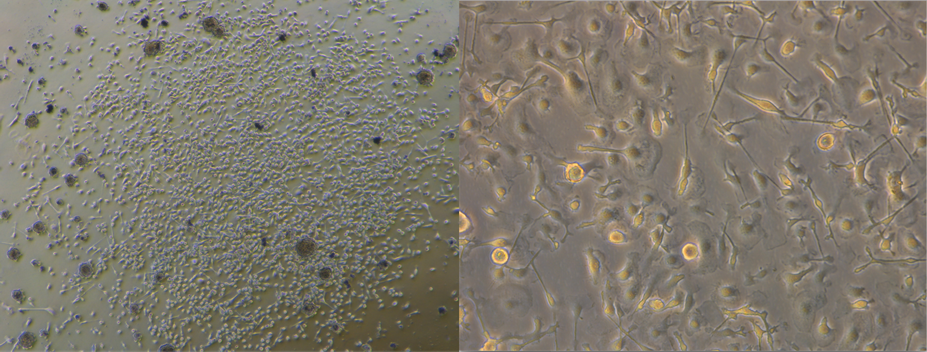
Left panel, A colony of microglia on non-TC tissue culture plastic 3 weeks after plating Right panel, live image of microglia before passaging (microglia verified using Iba1)
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
