Advanced Search
Sample preparation for light sheet microscopy
Last updated date: Oct 17, 2025 Views: 14 Forks: 0
I have experience with intravital imaging using the Zeiss Lightsheet Z.1 with zebrafish embryos and larvae at early developmental stages. In addition, I have also performed intravital imaging of living killifish, using a transparent line as described in the respective publication. For all experiments, I used the standard chamber (there is no extra-large chamber available, and it would not fit into the setup anyway), which means that the samples need to be small enough to fit into the chamber. The chamber was equipped with a Peltier element and a temperature sensor, which, together with the temperature modules belonging to our setup, ensured temperature control throughout the imaging process.
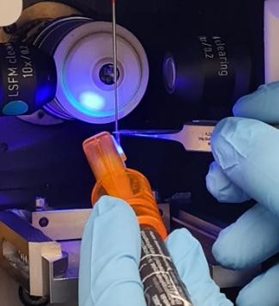
Regarding sample mounting of killifish at the age of 17 days post hatching, I gently attached a very small part of the caudal fin to a mounting support. For this purpose, I preferred to use a special UV light-curing resin with an applicator(Bondic UV Repair System), which allowed application of a minimal adhesive area. The resin was either applied onto a sample adapter available from Zeiss (see Quick Guide: Sample Holder Universal for Capillaries and the image below) or onto a customized mounting support that was modeled at the end of a standard capillary (see image below). For the experiments presented in the publication, I used the latter approach.
In addition, the following aspects regarding animal welfare should be noted:
To meet the requirements of animal welfare, I would first like to emphasize that before performing any imaging on live animals, we carried out initial trials using dead and fixed specimens. These preliminary tests were essential to establish suitable imaging conditions and to ensure that the settings were optimized without causing unnecessary stress to living animals.
During imaging, the animals’ vital parameters (including blood flow, heartbeat and opercular movement) were continuously monitored using the “Overview” and “Locate Sample” function implemented in the Lightsheet ZEN Black software (Zeiss). In case of a loss of vital functions (which did not occur during our experiments so far), the imaging procedure would have been immediately terminated, and the animal euthanized by immersion in an overdose of tricaine.
Furthermore, we ensured that no animal remained in the experimental procedure for longer than 25minutes in total (including anesthesia, mounting, and microscopy). After completion of the imaging process, the small, glued portion of the caudal fin was carefully cut off, and the animal was placed in a bath containing an overdose of tricaine. The fish was then euthanized and used for subsequent organ removal.
To make some of the provided information easier to understand,I have included the following pictures here:
- Sample adapters from Zeiss
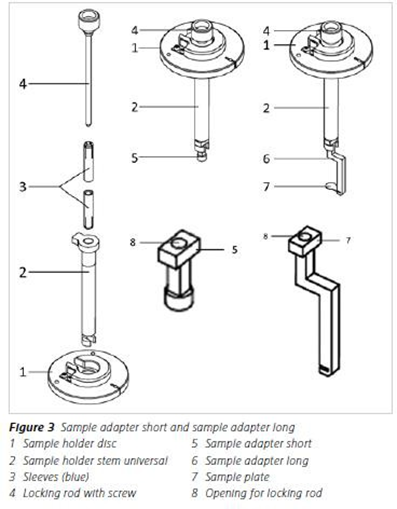
Sample adapter (short) in the correct position
(to start gluing the sample on it, before insertion of the chamber)

modeling a customized mounting support at the end of a capillary
(which fits in the standard sample holder)

gluing a small part of the caudal fin to the molded surface
- sample chamber
- equipped with a Peltier element
- filled with tank water and Tricaine
killifish inside was glued at the molded surface (at the end of the capillary)

Related files
 intravital_Lightsheet_imaging_BP_to EmiliaSeta.pdf
intravital_Lightsheet_imaging_BP_to EmiliaSeta.pdf - Krug, J, Englert, C, Perner, B, Albertz, C, Mörl, H and Hopfenmüller, V(2025). Sample preparation for light sheet microscopy. Bio-protocol Preprint. bio-protocol.org/prep2864.
- Krug, J., Perner, B., Albertz, C., Mörl, H., Hopfenmüller, V. L. and Englert, C.(2023). Generation of a transparent killifish line through multiplex CRISPR/Cas9mediated gene inactivation. eLife. DOI: 10.7554/eLife.81549
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link
