Advanced Search
Monitoring endocytosis of integral membrane proteins using western blot-based detection of biotinylated antibody-uptake
Last updated date: Sep 10, 2025 DOI: 10.21769/p2855 Views: 2 Forks: 0
Alexandra Graninger1, 2 and Prasanna Satpute-Krishnan2, *
1Neuroscience Program, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
2Department of Biochemistry and Molecular Biology, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
*For correspondence: prasanna.satpute@gmail.com
Abstract
The antibody-uptake assay is a commonly used technique to monitor endocytosis of integral membrane proteins including transmembrane and glycosylphosphatidylinositol-anchored proteins (GPI-APs). The antibody-uptake assay typically involves incubating live cells with fluorophore-conjugated antibodies directed against the extracellular domain of the integral membrane protein-of-interest. Antibody-uptake is then detected by flow cytometry or confocal microscopy. However, these detection modalities may be inaccessible to some labs or require extensive training to operate. Thus, we developed a facile and novel sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot-based approach to the antibody-uptake assay that exploits the strong affinity between biotin and streptavidin. Instead of incubating cells with fluorophore-conjugated antibodies to monitor antibody-uptake, our assay involves incubating cells with biotinylated antibodies, processing the cell lysates for western blot, and probing the membrane with detectably-conjugated streptavidin. From preparation to quantification, this protocol requires less hands-on time than other approaches, and is amenable to small-scale drug or siRNA screens. Here, we demonstrate the utility of our approach using the well-characterized misfolded GPI-AP, YFP-tagged C179A mutant of prion protein (YFP-PrP*), as our model substrate. YFP-PrP* constitutively traffics to the plasma membrane (PM), where it binds to anti-GFP antibody, and immediately undergoes endocytosis to lysosomes. To validate our protocol, we present measurements of antibody-uptake under conditions known to enhance or inhibit YFP-PrP*’s traffic to the PM. Using this assay, we present new evidence that, under certain conditions, YFP-PrP* is able to undergo degradation via a pathway that does not involve exposure on the cell surface.
Key features
- Summary: Incubate live cells with biotinylated primary antibody and a lysosomal degradation inhibitor, process lysates for western blot, and probe the blot with detectably-conjugated streptavidin.
- Fast, easy and semi-quantitative assay to test whether integral membrane proteins are degraded through pathways involving exposure on the plasma membrane.
- Conduct screens for small molecules, siRNAs, or conditions that promote or inhibit traffic of your protein-of-interest through the plasma membrane.
- Pair this protocol with a synchronized trafficking assay to detect changes in the rate of proteins traversing the plasma membrane.
Keywords: antibody-uptake, integral membrane protein trafficking, plasma membrane, RESET, internalization, transmembrane and GPI-anchored protein, secretory pathway, endocytosis
Graphical overview
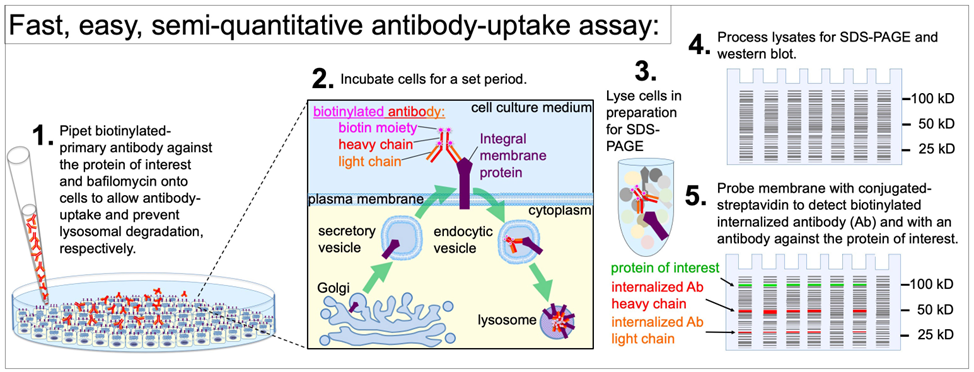
Background
Antibody-uptake utilizes the specific binding of primary antibodies to membrane proteins that are displayed on the plasma membrane (PM) and subsequently internalized via endocytosis. Integral membrane proteins, including transmembrane proteins and glycosylphosphatidylinositol (GPI)-anchored proteins, are synthesized in the endoplasmic reticulum (ER) and are able to traffic from the “cradle” to the “grave” by a variety of membrane trafficking pathways (Figure 1, dashed and solid arrows). Antibody-uptake assays exclusively reveal whether a population of the integral membrane proteins traffic to and are endocytosed from the PM (Figure 1, solid arrows). Determining whether a population of proteins traffic to the cell surface is essential for delineating the possible trafficking pathways an integral membrane protein may take prior to degradation. Additionally, antibody-uptake assays may inform our understanding of the signal transduction pathways that regulate the secretion and internalization of integral membrane protein receptors and channels.
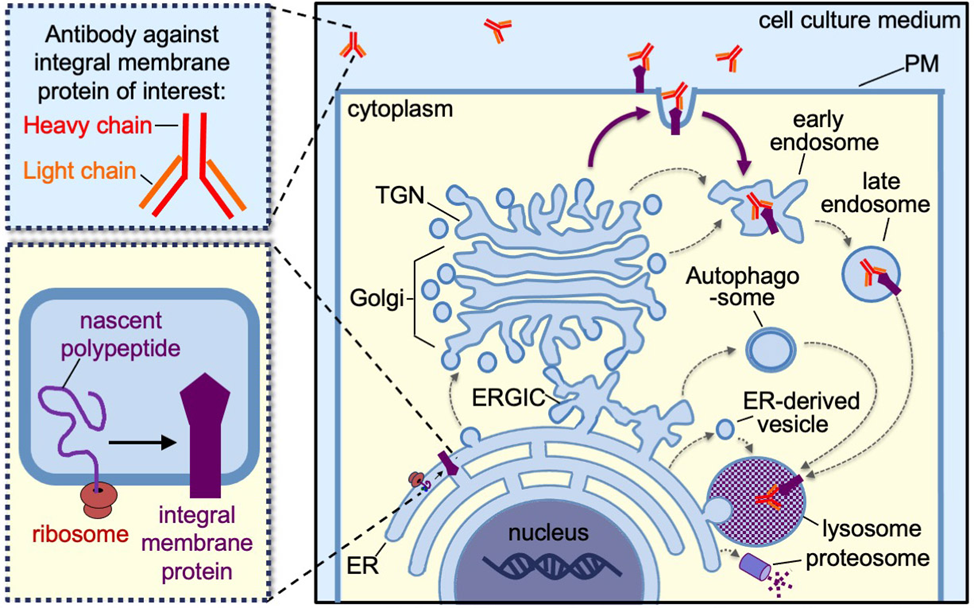
Figure 1: Schematic depicting the various trafficking pathways of an integral membrane protein from the endoplasmic reticulum, where it is synthesized (i.e. the “cradle”) to the lysosomes or proteosomes, where it is degraded (i.e. the “grave”). The thin black arrows indicate possible internal trafficking steps from one compartment to the next. Abbreviations include ER (endoplasmic reticulum), ERGIC (ER-Golgi-intermediate compartment), TGN (trans-Golgi network), and PM (plasma membrane). The thick dark purple arrows indicate the possible trafficking steps involving transit through the PM, which specifically can be detected by antibody-uptake assays.
Over the past 40 years, a variety of antibody-uptake approaches have been employed to monitor the internalization of integral membrane proteins from the PM. The primary antibodies are either directly conjugated to a detectable tag (e.g. fluorophore, gold particle, radiolabel) or are unlabeled and detected by incubating lysed or permeabilized fixed cells with detectable secondary antibodies. Immunofluorescence, immunogold electron microscopy, live-cell fluorescence imaging, flow cytometry, autoradiography and western blot have each been performed to detect the internalized primary antibodies [1-8]. Each of these approaches to detect and quantify antibody-uptake results pose distinct challenges. Quantifying fluorophore-conjugated antibody-uptake using microscopy-based techniques is time-consuming to measure fluorescence intensity in individual cells, requires imaging software for volumetric analysis and can be cost-prohibitive if a microscope is not readily available. Quantification of antibody-uptake of fluorophore-conjugated antibodies by flow cytometry allows for rapid readout of large populations of cells under various conditions, however many labs do not have ready access to a flow cytometer. Both fluorescence imaging-based and flow cytometry-based quantification may be thwarted by fluorescence quenching from fluorophore crowding or the pH of the endocytic vesicles and lysosomes. Quantification of radioactively-labeled antibody-uptake by autoradiography requires designated space and equipment for radioactive work. Microscopy, flow cytometry and radioactive work all require extensive training. By contrast, SDS-PAGE followed by western blot-based protocols using secondary antibodies to detect internalized primary antibodies utilize relatively inexpensive and standard lab equipment, require less training, and can be completed in 1 or 2 workdays. However, the standard western blot approach using primary and secondary antibodies has not demonstrated to provide a clear, reliable or measurable readout for antibody-uptake beyond a binary yes/no [1,7], and did not gain traction in the cell biology field. This may be in part because detection with secondary antibodies on western blot membranes depends on the internalized primary antibody maintaining antigenicity during denaturation in the lysosomes and during the subsequent cell lysis and SDS-PAGE process.
To address these practical, technical and biological limitations with standard antibody-uptake, we developed a novel approach that builds on the advantages of SDS-PAGE and western blot. Our approach exploits the extraordinarily high binding specificity and affinity between biotin and streptavidin [9-11]. The bond between streptavidin and biotin-conjugated molecules is one of the strongest naturally existing non-covalent bonds, with an equilibrium dissociation constant (KD) between 10−14-10−16 M [12,13]. This is stronger than most antibody-antigen interactions, which were reported by the Abcam company and academic investigators to have a KD between 10−6-10−9 M or, in the case of very high affinity antibodies, to have a KD of 10−12 M [14-17]. Our approach involves incubating live cells with biotinylated primary antibodies, processing the cells for western blot, and detecting antibody-uptake by probing the western blot membrane with either HRP-conjugated or fluorophore-conjugated streptavidin. Biotinylated primary antibodies are available commercially or can be easily generated using biotinylation kits. Additionally, a wide selection of labeled streptavidin is commercially available. Unlike microscopy-based antibody-uptake assays that require image analysis on a cell-by-cell basis, the western blot-based antibody-uptake approach produces population-level results that are quickly quantifiable through mean band intensities normalized against the total protein in the lysates. The expression level of the target integral membrane protein can be cross-correlated with antibody-uptake on the same western blot membrane by employing standard western blotting to probe for the integral membrane protein-of-interest. An additional advantage of our western-blot based approach is that cell lysates and western blot membranes can be preserved for extended periods at -20°C.
To demonstrate the utility of this approach, we use a fluorescent protein (FP)-tagged variant of prion protein C179A (PrP*). Untagged PrP*, yellow fluorescent protein (YFP)-PrP*, cerulean fluorescent protein (CFP)-PrP* and green fluorescent protein (GFP)-PrP* are well-characterized to leave the ER, traffic to the PM and rapidly undergo endocytosis [18-22]. During steady-state conditions, PrP* undergoes ER-to-Golgi export via an ER stress-inducible pathway called “RESET,” which is short for “rapid ER stress-induced export” [19,20]. PrP* flux through RESET regulates its access to the PM [18,20]. For YFP-PrP*, the YFP-tag is derived from GFP and therefore is recognized by the same anti-GFP antibodies that bind GFP due to structural similarity [20,23]. The FP-tag, however, does not drive the trafficking of PrP*, but merely provides a detectable label to monitor PrP* trafficking by live-cell imaging [20]. Critically, YFP-PrP* or GFP-PrP*-expressing cells were previously shown to internalize anti-GFP antibodies based on specific affinity between the anti-GFP antibody and the YFP- or GFP-tag by confocal microscopy and flow cytometry-based approaches [18-21]. Here we present proof-of-principle experiments using anti-GFP antibodies to monitor YFP-PrP* traffic through the PM en route to lysosomes. However, we expect that this antibody-uptake protocol will be applicable to monitor any protein displaying an antigen on an extracellular domain for which there is a biotinylated primary antibody available.
Materials and reagents
Biological materials
Healthy cells. This protocol applies to any adherent, proliferating or differentiated cultured cells.
Recommendations:Include a positive control to verify that your biotinylated antibody binds to the antigen (i.e. your integral membrane protein-of-interest) in the context of the antibody-uptake conditions.
If there is no pre-existing evidence that your integral membrane protein-of-interest traffics to the PM, consider overexpressing a fusion protein to test the antigen-antibody interaction. This fusion protein would include the extracellular domain of your integral membrane protein-of-interest (i.e. the antigen) on a known PM-resident protein and function as a positive control.
To validate our protocol, we used the following cells for our positive controls:YFP-PrP* NRK cells: These normal rat kidney (NRK) cells stably express the well-characterized RESET substrate, YFP-PrP*, at physiological levels, similar to prion protein in mouse brain lysate [19,20]. YFP-PrP* includes an N-terminal prolactin signal sequence to ensure ER-translocation, a yellow fluorescent protein (YFP) tag, followed by the Syrian hamster prion protein (PrP) mature domain containing a C179A point mutation [20]. YFP-PrP* and GFP-PrP* were each previously shown to bind and internalize anti-GFP antibody upon accessing the PM [18-21].
YFP-PrP* NRK cells treated with thapsigargin (TG): TG-treatment of YFP-PrP* NRK cells was shown to increase the flux of YFP-PrP* traffic to the PM and antibody-uptake when compared to untreated YFP-PrP* NRK cells [20].
Include negative controls to determine baseline antibody-uptake. This can be cells treated with RNAi or CRISPR knockout technology to remove the integral membrane protein-of-interest, or cells treated with chemical or siRNA-based inhibitors of the secretory pathway to prevent the integral membrane protein-of-interest from accessing the PM.
To validate our protocol, we used the following cells for our negative controls:Normal Rat Kidney (NRK) cells: These untransfected cells serve as a negative control for anti-GFP antibody-uptake because they do not express an FP-tagged protein. They are the “parental” cell line from which the YFP-PrP* NRK stable cell line was derived. NRK cells were previously characterized as adherent cells [24].
YFP-CD3δ NRK cells: These NRK cells stably express YFP-CD3δ, a well-characterized ER-associated degradation (ERAD) substrate [25,26], at similar levels to the expression of YFP-PrP* in YFP-PrP* NRK cells [19,20]. They serve as a negative control because YFP-CD3δ would not be expected to undergo ER-export, but is instead be degraded at the ER [25,26].
YFP-PrP* NRK cells treated with BRD4780: BRD4780-treatment of YFP-PrP* NRK cells was shown to inhibit RESET of YFP-PrP* [18]. Thus, BRD4780 would be expected to block traffic to the PM and thereby prevent anti-GFP antibody-uptake.
YFP-PrP* NRK cells treated without antibody in media: These cells would not be expected to show a streptavidin-binding bands by western blot, because no antibodies would have been added in the medium for the cells to uptake.
Reagents
Cell culture reagents:
Cell culture medium.
We used the following cell culture medium components:Dulbecco’s Modified Eagles Medium (DMEM, Corning, catalog number: 17-205-CV)
Fetal Bovine Serum (FBS, Corning, catalog number: 35-011-CV)
L-Glutamine (Corning, catalog number: 25-005-CL).
Biotinylated primary antibody.
We used biotinylated goat anti-GFP antibody (Genetex, catalog number: GTX26658).
Optional: inducers or inhibitors that promote or block the protein-of-interest’s traffic to the PM.
We used the following:Thapsigargin (Sigma, catalog number: 586005)
BRD4780, also called AGN192403 hydrochloride (Tocris, catalog number: 1072).
Lysosomal degradation inhibitor to prevent degradation of biotinylated antibody and protein of interest.
We used Bafilomycin A1 (LC Laboratories, catalog number: B-1080).Wash buffer to wash away excess non-internalized antibody.
We used phosphate buffered saline (PBS), pH 7.4 (KD Medical, catalog number: RGF-3210)
Western blot reagents:
2X Laemmli sample buffer.
We used ready-made 2X sample buffer (Bio-Rad, catalog number: 1610737).Reducing agent to break disulfide bonds for optimal SDS PAGE and western blot.
We used tris(2-carboxyethyl)phosphine (TCEP, Sigma CAS number: 51805-45-9, catalog number: 646547).Protein gels for SDS-PAGE.
We used ready-made 12% Mini-PROTEAN® TGX Stain-Free™ 15 well gels (Bio-Rad, catalog number: 4568046).Molecular Weight ladder.
We used Precision Plus Protein™ All Blue PreStained Protein Standards, (Bio-Rad, catalog number: 1610373)Transfer reagents for western blot.
We used transfer kit including PVDF membrane (low fluorescence, 0.45 µm) that requires ethanol for activation, filter paper, and 5X transfer buffer (Bio-Rad, catalog number: 1704275) that requires supplementation with ethanol, 200 Proof (Sigma, catalog number: 459829-2L)Gel running buffer.
We used Tris Glycine SDS, pH 8.3 (Santa Cruz Biotechnology, catalog number: sc-296527)Membrane washing buffer.
We used Tris Buffered Saline 10X, pH 7.4 (Santa Cruz Biotechnology, catalog number: sc-362308) supplemented with Tween® 20 (Sigma, catalog number: P1379-1L)Blocking buffer.
We used EveryBlot Blocking Buffer, (Bio-Rad, catalog number: 12010020)Labeled streptavidin to detect the internalized biotinylated primary antibody.
We used DyLight800-conjugated streptavidin (Streptavidin-DyLight800, Bio-Rad, catalog number: STAR152D800GA). An alternate is HRP-conjugated Streptavidin (Proteintech, catalog number: SA-0000-10).Enhanced chemiluminescence reagents to detect HRP-conjugated streptavidin or secondary antibodies.
We used Chemiluminescence, long duration (Clarity ECL, Bio-Rad catalog number 1705061) and Chemiluminescence, high sensitivity (Clarity Max ECL, Bio-Rad catalog number 1705062).
Solutions
Complete DMEM: 10% FBS and 2mM L-glutamine in DMEM
Thapsigargin (TG): 0.1 μM TG in complete DMEM
- BRD4780 (also called in AGN192403 hydrochloride): 100 μM in complete DMEM
Bafilomycin A1: 500nM Bafilomycin A1 in complete DMEM
Biotinylated Goat anti-GFP antibody: 1 μg/ml in complete DMEM
2X Laemmli sample buffer (2XSB) + tris(2-carboxyethyl)phosphine (TCEP): 5mM TCEP in 2XSB
Tris Glycine SDS: 0.025M Tris, 0.192M glycine, and 0.1% SDS at pH 8.3
TBST: 250 mM Tris, 27 mM KCl, 1.37 M NaCl at pH 7.4 with 0.1% Tween® 20
Streptavidin-DyLight800: Diluted 1:5000 in EveryBlot Bio-Rad blocking buffer
Laboratory supplies
10 cm tissue culture-treated dish (VWR, catalog number: 25382-428)
6-well tissue culture treated Plates, sterile, flat-bottom wells (Genesee, catalog number: 25-105MP)
Gel loading tips, 1–200 µl pipet tip for gel loading, (Bio-Rad, catalog number: 12021140)
Boil-proof microfuge tubes (Genesee Scientific, catalog number: 24-282C)
Equipment
Gel electrophoresis apparatus.
We used Mini-PROTEAN® Tetra Electrode Assembly (Bio-Rad, catalog number: 1658037).Western blot transfer apparatus.
We used Trans-Blot Turbo Transfer System (Bio-Rad, catalog number: 1704150).- Gel and western blot documentation apparatus.
We used ChemiDoc™ MP Imaging System (Bio-Rad, catalog number: 12003154).
Software
- FIJI “Fiji is just ImageJ” (National Institutes of Health)
Excel (Microsoft)
Prism (Graphpad)
Procedure
A. Cell Culture and Preparation
Thaw cells into 10 cm tissue culture-treated dish in standard cell culture medium.
To validate our protocol, we used NRK, YFP-PrP* NRK and YFP-CD3δ NRK cells cultured in DMEM supplemented with 10% Fetal Bovine Serum (FBS) and L-Glutamine. We did not include penicillin/streptomycin. We cultured the cells at 37°C in 5% CO2 and maintained them in the growth phase.Split the cells into a multi-well dish. Depending on the cell type, split them at the optimal confluency for growth or differentiation.
For our validation experiments, we used a 6-well dish. We split cells so they were at 35% confluency, and allowed the cells to double over 24 h to approximately 70% confluency.
B. Treatment with biotinylated antibody diluted in culture media and cell lysis
Check cells on the day of the experiment to ensure healthy growth and confluency.
We performed our experiments on cells that were 70% confluent in order to avoid over-crowding and contact inhibition.Prepare all media in advance of treating cells.
We prepared the following media.“+Baf” condition: Complete medium with 500 nM Bafilomycin A1.
“+Baf +Ab” condition: Complete medium with 500 nM Bafilomycin A1 and 1 µg/ml biotinylated antibody.
“+Baf +TG +Ab“ condition: Complete medium with 500 nM Bafilomycin A1, 0.1 µM TG, and 1 µg/ml biotinylated antibody.
“BRD4780 pretreatment” media: Complete medium with 100 μM BRD4780.
“+BRD4780 +Baf +Ab“ condition: Complete medium with 100 µM BRD4780 added to cells for a 3 hour pretreatment, followed by the addition of 500 nM Bafilomycin A1, and 1 µg/ml biotinylated antibody.
“+BRD4780 +Baf +BRD4780 +TG +Ab“ condition: Complete medium with 100 µM BRD4780 added to cells for a 3 hour pretreatment, followed by the addition of 500 nM Bafilomycin A1, 0.1 µM TG and 1 µg/ml biotinylated antibody.
Replace existing culture media with prepared media containing bafilomycin, +/- biotinylated antibody, +/- drug modulators.
Note: We added Bafilomyin A1 to cells. Bafilomycin A1 is a potent inhibitor of vacuolar ATPases that prevents acidification of lysosomes and consequently prevents lysosomal degradation [27] Blocking lysosomal degradation of the internalized biotinylated antibody is necessary for subsequent western blot analysis.
Note: Specifically for “+BRD4780” treatments, we pretreated cells with 100 µM BRD4780 for 3 h to induce degradation of TMED9, which we previously showed causes complete inhibition of RESET [18]. After the 3 h pretreatment with BRD4780, the cells were co-incubated with Bafilomycin A1 and biotinylated anti-GFP antibody or Bafilomycin A1, TG and biotinylated anti-GFP antibody for an additional 2 h.Incubate cells for 2 h at 37 °C, 5% CO2.
Remove cells from incubator and aspirate all culture media.
Wash cells three times with room temperature PBS prechilled to 4 °C.
Critical: During each of the three washes, rock dish back-and-forth three times to ensure removal of all biotinylated antibody remaining in culture media.Add lysis buffer to cells, scrape with precut pipette tips and collect lysates in prelabeled boil-proof microfuge tubes.
Note: We used 200 µl 2xSB+TCEP pipetted directly into each well of the 6-well plates to lyse cells.
We precut p200 pipette tips using standard desk scissors at approximately the 10ul point at a 45° angle to increase the bore size and the surface area for scraping the cells. However, the key is to scrape the surface of the well and gather the lysate together to move it into boil-proof microfuge tubes.Boil the lysates for 1 min and vortex for 5 seconds three times in order to break up all of the DNA and solubilize the lysate.
Store lysates at -20 °C.
C. Western Blot
Note: Western blot procedure may be conducted based upon individual lab’s specific western blot workflows.
Allow frozen samples stored at -20 °C to thaw at room temperature (approximately 5 min).
Vortex all samples briefly (approximately 5 seconds) to ensure homogenous mixture.
Note: If samples were collected in lysis buffer (i.e., RIPA buffer) instead of directly in sample buffer (i.e., Laemmli buffer with reducing agent), add appropriate volumes of western blot sample buffer to each sample.Load samples into prepared polyacrylamide gel in gel running apparatus.
Note: We use Bio-Rad Mini-PROTEAN Protein electrophoresis equipment and Tris Glycine SDS as running buffer.
Critical: Ensure equal or near-equal loading of total protein across all lanes. We do this by seeding the same number of cells in each well at the start of the experiment. Alternatively, immediately after cell lysis, one can employ total protein normalization techniques (e.g. the BCA assay).Run gel for approximately 60-75 min at 100V.
Note: If using Bio-Rad Stain-Free™ system, activate and collect an image of Stain-Free™ gel to detect total protein.Transfer the gel to an activated PVDF membrane for blotting.
Note: After transfer, image the Stain-Free™ blot. The Stain-Free™ system provides an image of total protein, which we use as a loading control. If the Stain-Free™ system is not available, stain the blot with Ponceau S to image total protein loaded into each lane. Ponceau S washes away during the following steps. We recommend using a low fluorescence membrane to improve western blot signal, especially when using fluorescent secondary antibodies.Block membrane.
Note: We used Bio-Rad EveryBlot blocking buffer for 10 min at room temperature on gentle shaker. An alternate is 2% bovine serum albumin (biotin-free) in TBST.
Critical: Do not use milk as a blocking buffer. Milk contains biotin naturally which may interfere with streptavidin binding and blotting procedure.Probe blot with streptavidin that was pre-conjugated to a detectable label. Dilute the streptavidin in blocking buffer for 1 h at room temperature with gentle shaking.
Note: We used DyLight800-conjugated streptavidin and diluted it at 1:5000 in Bio-Rad EveryBlot blocking buffer. An alternative is HRP-conjugated streptavidin. An advantage of probing DyLight800-conjugated streptavidin is that the same blot may be with reprobed with an antibody against your protein-of-interest and detected using an HRP-conjugated secondary with no cross talk between western blot images. Alternatively, run the lysates on two identical gels, and probe one with HRP-streptavidin, and the other with the primary antibody against your protein-of-interest for standard western blot procedure using a HRP-conjugated secondary antibody.Wash the blot 3 times for 10 min each time with TBST.
Image the streptavidin blot with gel documentation imaging system, selecting exposure times that allow the light collected to remain within the camera’s dynamic range.
Note: We use the Bio-Rad ChemiDoc MP gel documentation imaging system to collect images of the DyLight 800 or chemiluminescence. This system displays an indicator when pixels are oversaturated due to excessively long exposure times.
Optional: Reprobe using standard western blot procedure for your integral membrane protein-of-interest.
Note: Avoid saturating the pixels during image collection. Stay within the camera’s dynamic range of the gel documentation system because saturated pixels are not quantifiable.
Data analysis
To measure western blot bands, we utilized the following step-by-step procedure:
After collecting unsaturated western blot images, download .tif images onto a computer or laptop with Fiji installed.
Open .tif file corresponding to streptavidin blot in Fiji.
Note: If needed, rotate blot to straighten it. Ensure bands appear white and background appears black by inverting if needed.Draw a rectangle (bounding box) that encompasses the largest band of interest in the blot (see Figure 2).
Save as region of interest (ROI).
Measure the mean gray value (MGV) of each band of interest using the saved ROI. In Fiji, drag the ROI rectangle over each band and press “M” to measure.
Note: Mean gray value is the average intensity of all the pixels within a given area. Therefore, MGV is a measure of blot band intensity in this case.Measure the mean gray value (MGV) of the background for each lane by measuring 2 bounding boxes below the band of interest (Figure 2).
Copy measurements from Fiji to Excel worksheet to perform calculations.
Generate background-subtracted MGV for each band of interest by subtracting background MGV from back of interest MGV for each lane.
To normalize to total protein loaded, repeat steps 2-8 with .tif file of loading control (stain free blot, Ponceau blot, or housekeeping protein blot). Then divide background-subtracted MGV of band of interest by background-subtracted loading control. This is the normalized, background-subtracted MGV of the band of interest.
Note: We used Bio-Rad Stain-Free™ blot system to show total protein loaded.To normalize by one condition and therefore reflect all other conditions as a multiple, fraction or percentage of the baseline condition, divide the background-subtracted MGV of the band of interest of each lane by the baseline condition.
Note: In our experiments, YFP-PrP* +Baf +Ab is our baseline condition. The internalized antibody heavy-chain band for this condition is expressed as 1 on all graphs. Therefore, the internalized antibody heavy-chain bands for all other conditions are expressed as a multiple, fraction or percentage of this condition. For example, 2.5 represents 250% the intensity of the internalized antibody heavy-chain band YFP-PrP* +Baf +Ab. Similarly, 0.10 represents 10%.After normalizing, statistical testing can be run by transferring the data from Excel into GraphPad Prism.
Note: General familiarity using Prism GraphPad and background in statistical analysis is helpful for conducting quantifications. It is recommended to conduct 3 biological replicates for each quantified experiment.
To conduct statistical testing on analyzed data, we followed the following step-by-step procedure:
Before beginning, it is important to understand that data generated from western blots is semi-quantitative. Therefore, protein expressions are only relative to each other and can only be described as a fold-change ‘more’ or ‘less’ than another condition. Due to this semi-quantitative nature and small sample sizes (typically n = 3 replicates), careful consideration must be taken before performing any statistical testing.
After transferring data from Excel to Prism, plot data with appropriate descriptive statistics to examine variance and skew. It is important to evaluate variance and skew to determine appropriate statistical testing (if any).
Conduct statistical tests and multiple comparisons if applicable.
Note: We have chosen to compare all conditions to our control condition (YFP-PrP* +Baf +Ab). We have decided to use an ordinary one-way ANOVA with Dunnett’s correction for multiple tests. We chose this test because our data is relatively normally distributed and the variances are approximately equal across experimental conditions. Alternatively, if comparing only two groups, a t-test could be used. If the data does not have equal variance and is skewed, then comparing confidence intervals may be more appropriate. Additionally, a one sample t test or Wilcoxon test to compare one experiment condition to a control condition with a set value (i.e., “1” after normalization) could be used.If statistical significance is found, include asterisks to denote finding on future graphs.
Note: Specific statistical tests that we used are denoted in the figure legend accompanying each graph. Importantly, some differences may be statistically insignificant but still demonstrate a meaningful, biological difference.
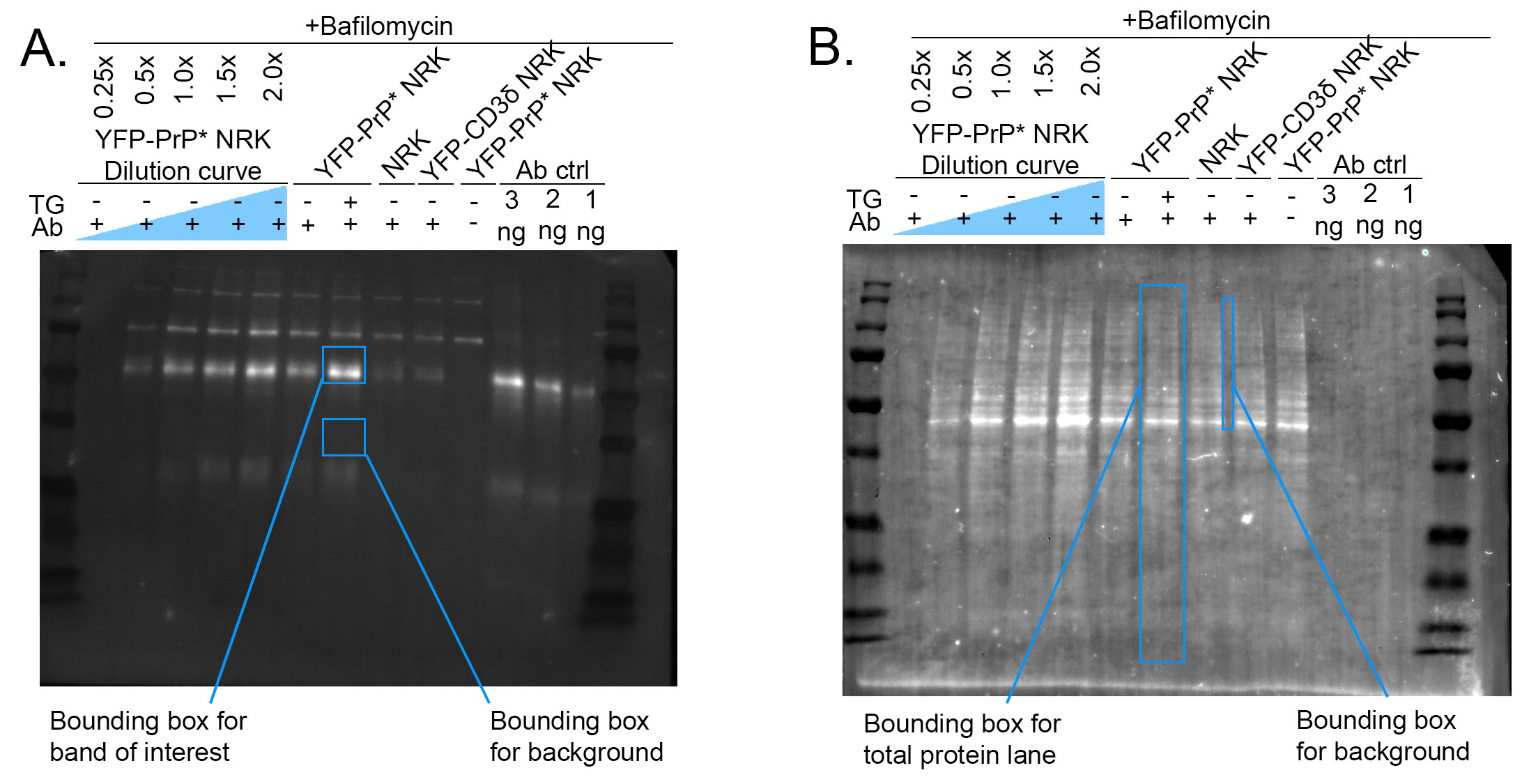
Figure 2: Example of western blot band and background measurements. (A) Depiction of the rectangular bounding box used to measure the mean gray value of western blot bands of interest and background measurements. (B) Depiction of the rectangular bounding box used to measure the mean gray value of stain free blot bands of interest representing total protein loaded and background measurements.
Validation of protocol
In order to interrogate and validate our new protocol, we have taken advantage of two key features of PrP* trafficking. First, tagged variants of PrP* were previously documented to be rapidly endocytosed upon arriving to the PM [20,21]. Second, rates of PrP*-trafficking from the ER to the PM were shown to be increased or decreased by small molecule drugs [18-20]. Thus, by controlling PrP*-trafficking from the ER to the PM, we are able to control antibody-uptake in predictable ways.
During steady-state conditions, YFP-PrP* is slowly released from the ER-resident chaperone, calnexin, for secretion to the PM via an ER-stress inducible ER-to-Golgi export pathway called “RESET,” which is short for “rapid ER-stress induced export (RESET)” [19,20]. At the PM, YFP-PrP* gets rapidly endocytosed and traffics to lysosomes [20]. Flux through the RESET pathway and subsequent access to the PM are rapidly enhanced with the chemical ER stressor, thapsigargin (TG), which triggers dissociation of YFP-PrP* from calnexin [18-20]. Expression of the p24-family members TMED9, TMED2 and TMP21, is required for the ER-to-Golgi export of YFP- or GFP-PrP* via RESET [18,20,21]. BRD4780, which degrades the essential RESET factors TMED9, TMED2 and TMP21 [18,28-31], inhibits RESET of YFP-PrP* [18].
To demonstrate that our western blot-based approach to monitor antibody-uptake provides a measurable readout for protein trafficking through the PM, we exploit the following established discoveries:
Untransfected “parental” NRK cells do not efficiently internalize anti-GFP antibody, while YFP-PrP* NRK cells do internalize anti-GFP antibody (Figure 3A-B) [20]. This implicates the direct interaction between anti-GFP antibody and the cell surface-exposed YFP-tag of YFP-PrP*. Thus, we expect to see no anti-GFP antibody-uptake in parental NRK cells (Figure 3A), but some antibody-uptake in YFP-PrP* NRK cells (Figure 3B).
Increasing the flux of YFP-PrP* through the RESET pathway with TG-treatment consequently increases the rate of anti-GFP antibody-uptake (Figure 3C) [20]. Thus, we expect to see an increase in anti-GFP antibody-uptake by YFP-PrP* NRK cells that are treated with TG (Figure 3C) when compared to untreated YFP-PrP* cells (Figure 3B).
BRD4780 inhibits the traffic of YFP-PrP* through the RESET pathway [18]. Thus, we expect that BRD4780-treatment of YFP-PrP* NRK cells would block anti-GFP antibody-uptake during steady-state or TG-induced ER stress conditions (Figure 3D-E).
YFP-tagged version of the classic ERAD substrate CD3δ, called “YFP-CD3δ” [32], remains in the ER for constitutive or TG-induced ERAD [25,32-34]. Therefore, we expect that YFP-CD3δ would not internalize anti-GFP antibodies in untreated or TG-treated YFP-CD3δ NRK cells (Figure 3F-G).
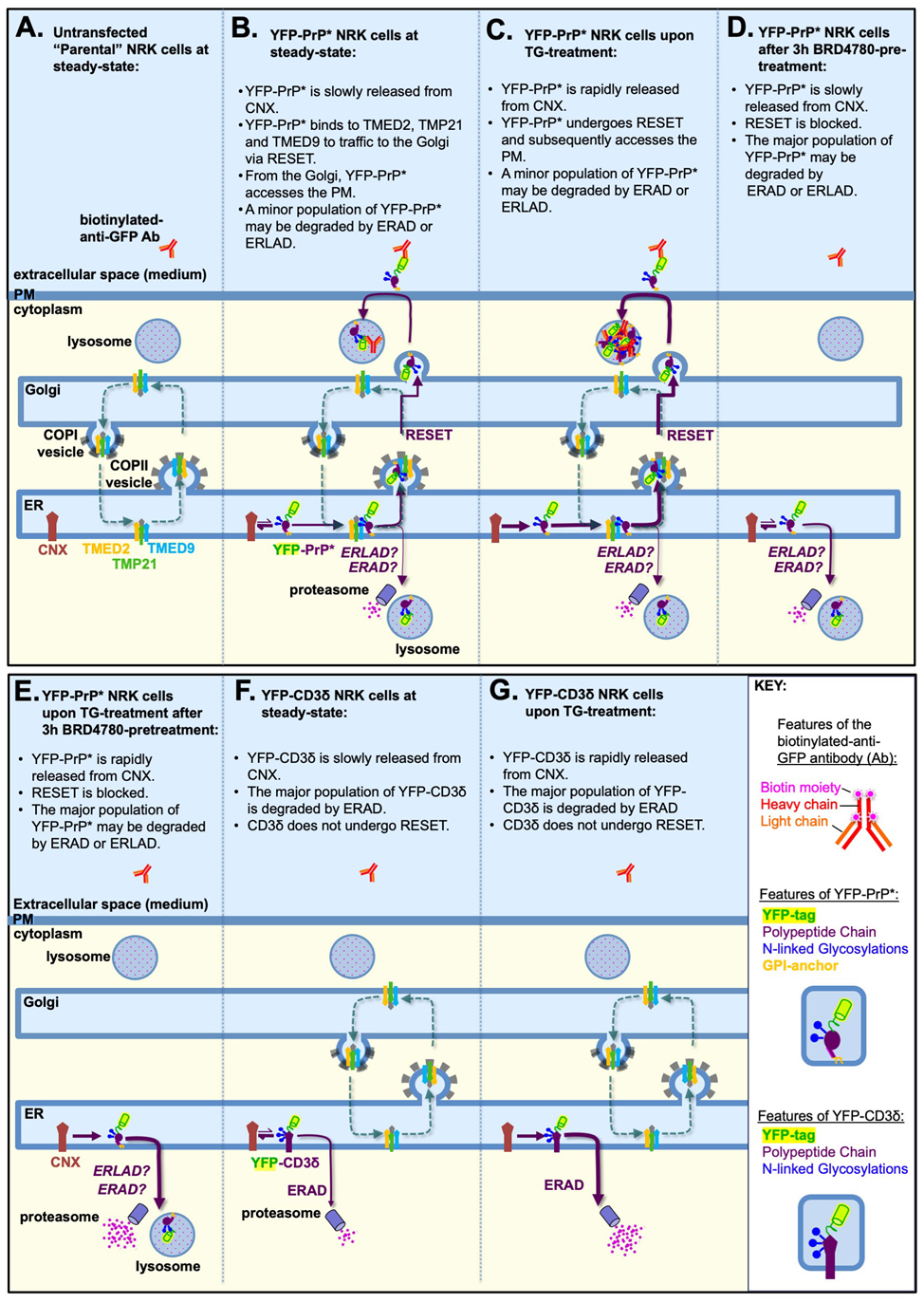
Figure 3: Model depicting the expected antibody-uptake results based on previously published experiments. (A-F) The p24-family members, TMED9, TMED2 and TMP21, hetero-oligomerize with each other and possibly other p24-family members, and constitutively cycle between the ER and Golgi [35-37], unless they are selectively degraded upon BRD4780-treatment [28-30]. Calnexin (CNX) is an ER-resident chaperone [38-41]. (B-E) The italicized “ERAD? ERLAD?” indicate that a role for these degradation pathways was not previously detected or investigated. (A) Parental (untransfected) NRK cells would not be expected internalize detectable levels of anti-GFP antibodies because they do not express an FP (e.g. YFP) tagged integral membrane protein (e.g. YFP-PrP*). (B) During steady-state conditions in YFP-PrP* NRK cells, CNX strongly associates with the misfolded GPI-anchored protein YFP-PrP*. Occasionally CNX releases YFP-PrP* and YFP-PrP* engages with the p24-family members to undergo ER-to-Golgi export via the RESET pathway. From the Golgi, YFP-PrP* gains access to the cell surface where it can bind and internalize anti-GFP antibodies. (C) Thapsigargin (TG)-treatment induces rapid release of the entire population of YFP-PrP* from CNX for the RESET pathway, allowing for increased anti-GFP antibody-uptake. (D) 3-hour (3h) BRD4780-pretreatment shuts down the RESET pathway by inducing the degradation of the requisite RESET factors, TMED9, TMED2 and TMP21. Thus, BRD4780-pretreatment would be expected to block constitutive turnover of YFP-PrP* via RESET and thereby prevent anti-GFP antibody-uptake. (E) 3h BRD4780-pretreatment of YFP-PrP* NRK cells shuts down RESET. Thus, co-incubation of BRD4780-pretreated YFP-PrP* NRK cells with TG, which forces YFP-PrP* release from CNX, would be expected to increase the rate of YFP-PrP* degradation via an alternate internal pathway. (F) During steady-state conditions, CNX occasionally releases the integral membrane protein, YFP-CD3δ. However, YFP-CD3δ is a well-characterized ERAD substrate that was shown not to undergo ER-export or RESET. Thus, we expect YFP-CD3δ would not access the cell surface and internalize anti-GFP antibody. (G) TG-treatment is known to increase the rate of ERAD of YFP-CD3δ. YFP-CD3δ does not undergo RESET and therefore would not be expected to internalize anti-GFP antibody.
To validate our protocol, we first tested whether internalized anti-GFP antibody can be detected in cells. We compared cell lysates of YFP-PrP* NRK cells treated with Bafilomycin A1 with or without anti-GFP antibody diluted in media (+Baf +Ab vs. +Baf –Ab; Figure 4, lanes 1 and 2) against anti-GFP antibody directly dissolved into sample buffer (Figure 4, lane 3). We probed the blot with an antibody against GFP to detect YFP-PrP*, and with Streptavidin-DyLight800 to detect the biotinylated antibody heavy and light chains. Critically, we were able to detect the antibody bands specifically in the lanes where the antibody was included. Taken together, biotinylated primary antibody that has been internalized into cells is readily detectable by SDS-PAGE and western blot when using streptavidin to probe the blot (Figure 4).
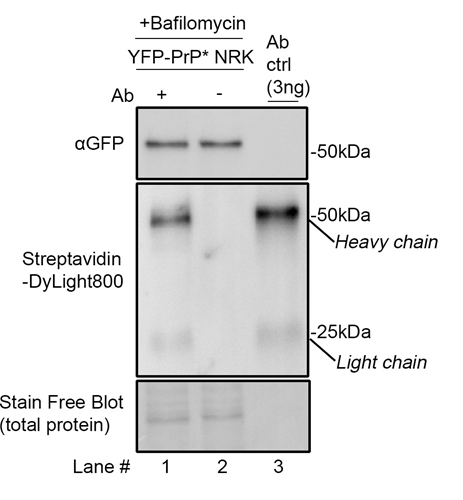
Figure 4: Western blot demonstrating the detection of internalized biotinylated anti-GFP antibody with streptavidin. YFP-PrP* NRK cells were incubated for 2h with 500 nM Bafilomycin A1 with 1 μg/mL antibody (Ab) (lane 1) or, as a negative control, without antibody (lane 2). To generate the antibody control lane (”Ab ctrl”), the biotinylated anti-GFP antibody was diluted directly into sample buffer to a final concentration of 0.2 μg/ml and then 15 μL was run on the gel, resulting in 3 ng loaded into the gel. The same membrane was imaged for total protein using the Stain-Free™ system, then probed with Streptavidin-DyLight800, and a homemade rabbit anti-GFP polyclonal antibody, which was previously described [19,20], and detected using HRP-conjugated anti-rabbit secondary antibody.
Next, we tested whether we could detect differences in anti-GFP antibody-uptake by YFP-PrP* NRK cells, untransfected parental NRK cells, and YFP-CD3δ NRK cells based on the trafficking concepts diagrammed in Figure 3. To provide an internal measurement guide, we included a dilution curve of YFP-PrP* NRK cell lysates from cells that were incubated with Bafilomycin A1 and anti-GFP antibody during steady-state conditions (Figure 5A-B, lanes 1-5). We generated this dilution curve by diluting lysate in 2xSB+TCEP prior to running it on the gel. Since heavy- and light-chains are stoichiometric in functional antibodies [42,43], we opted to measure the band intensities of only the 50 kDa biotinylated heavy chains that were stained by Streptavidin-DyLight800. We normalized the measurements against the 1X dilution of the cell lysate. This dilution curve reveals that while this western blot approach is semi-quantitative, it is useful to estimate decreases or increases in internalized antibody levels. Furthermore, using this approach, we detected the expected increase in anti-GFP antibody-uptake that occurs upon the additional treatment of YFP-PrP* NRK cells with TG. Our results suggest that TG-treatment increased anti-GFP antibody-uptake (Figure 5A-B, compare lanes 6 and 7). Critically, TG-treatment only induced an obvious increase in anti-GFP antibody-uptake by YFP-PrP* NRK cells, but not by the negative controls, including untransfected parental NRK cells or the YFP-CD3δ-expressing NRK cells (Figure 5A-B, compare lanes 6 and 7 to 8-11). This verifies the specificity of the anti-GFP antibody for the extracellularly-exposed YFP-tag of the YFP-PrP*.
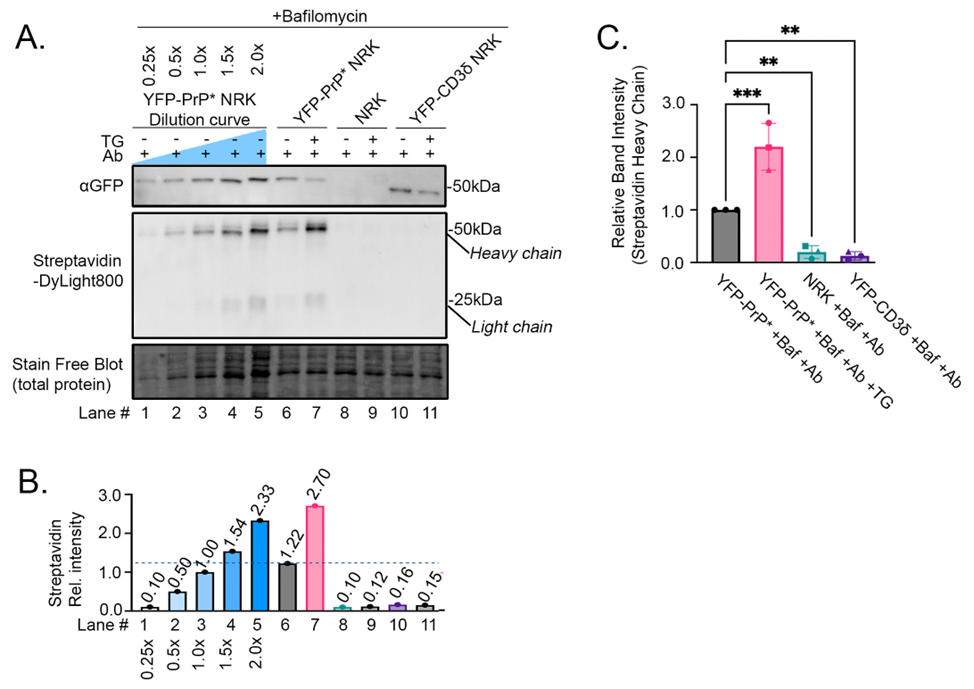
Figure 5: Proof-of-principle experiment demonstrating the expected differences in anti-GFP antibody-uptake as detected by our western blot-based approach. (A) Western blot of YFP-PrP* NRK, parental NRK, or YFP-CD3δ NRK cell lysates from cells that were incubated for 2 h with 500 nM Bafilomycin A1, 1μg/mL antibody (Ab) and 0.1μM TG as indicated. Lanes 1-5 represent a dilution curve of the YFP-PrP* NRK cell lysates that were incubated for 2 hours with 500 nM Bafilomycin A1 and 1μg/mL Ab. The same membrane was imaged for total protein using the Stain-Free™ system, then probed with Streptavidin-DyLight800 and rabbit anti-GFP polyclonal antibody followed by HRP-conjugated anti-rabbit secondary antibody. (B) Quantification of the Streptavidin-DyLight800-bound heavy chain bands of the internalized biotinylated anti-GFP antibody. All of the band intensities were normalized against the 1X dilution of the YFP-PrP* NRK cell lysates. (C) Triplicate quantification of Streptavidin-DyLight800-bound heavy chain bands of the internalized biotinylated anti-GFP antibody. Data is described as means and standard deviations. All of the band intensities were normalized against the total protein loaded in the Stain-Free™ blot and also the 1X dilution of the YFP-PrP* NRK cell lysates. Symbols represent each of the 3 trials. The circles correspond to the blot shown in (A). Means and standard deviations for each condition are as follows: YFP-PrP* +Baf +Ab, 1.000+/- 0.000; YFP-PrP* +Baf +Ab +TG, 2.202 +/- 0.444; Parental NRK +Baf +Ab, 0.199 +/- 0.123; YFP-CD3δ NRK +Baf +Ab, 0.126 +/- 0.080. Asterisks indicate statistically significantly different from YFP-PrP* +Baf +Ab (p<0.05) using an ordinary one-way ANOVA test comparing each group to the control condition (YFP-PrP* +Baf +Ab) with Dunnett’s correction for multiple comparisons.
After determining that the anti-GFP antibody-uptake by YFP-PrP* NRK cells is specifically due to the cell-surface exposure of the YFP-tag on YFP-PrP* in Figure 5A-B, we repeated a subset of the experimental conditions in triplicate for quantification (Figure 5C). Specifically, we measured anti-GFP antibody-uptake for YFP-PrP* NRK cells that were cultured with Bafilomycin A1 and anti-GFP antibodies (Ab) in the absence of TG (“YFP-PrP* +Baf +Ab”) or presence of TG (“YFP-PrP* +Baf +Ab +TG”). We measured two negative controls, parental untransfected and YFP-CD3δ NRK cells cultured with Bafilomycin A1 and anti-GFP antibodies (“NRK +Baf +Ab” and “YFP-CD3δ +Baf +Ab”, respectively). Triplicate quantifications revealed that in YFP-PrP* NRK cells, approximately double the amount of anti-GFP antibodies are internalized upon TG-treatment. This suggests that twice as many molecules of YFP-PrP* traffic through the PM within the 2-hour period when RESET is induced with TG. The negative controls demonstrated a low level of non-specific antibody-uptake during the 2-hour incubation. Taken together, we have demonstrated the utility of our western blot-based approach to detect specific antibody-uptake and increased antibody-uptake in response to a small molecule that promotes traffic of an integral membrane protein-of-interest to the PM (Figure 5).
We next set out to demonstrate the utility of our protocol to detect inhibition YFP-PrP* trafficking to the PM in response to a small molecule drug. As previously published, 3 hours of pretreatment with BRD4780 induces degradation of TMED9 and blocks traffic of YFP-PrP* through the RESET pathway [18]. Thus, we expected that a 3-hour BRD4780-pretreatment would block the consequent anti-GFP antibody-uptake during steady-state and TG-treatment conditions (Figure 3D-E). BRD4780-pretreatment visibly induced the degradation of TMED9 (Figure 6A, TMED9-blot, compare lanes 6-7 with 8-9). To provide an internal measurement guide for antibody-uptake, we included a dilution curve of YFP-PrP* NRK cell lysates from cells that were incubated with Bafilomycin A1 and anti-GFP antibody (Figure 6A, lanes 1-5). We measured the band intensities of the 50 kDa biotinylated heavy chains that were stained by Streptavidin-DyLight800 and normalized the measurements against the 1X dilution of the cell lysate (Figure 6A-B, Streptavidin-DyLight800 blot, compare lanes 1-9 with lane 3). As predicted for conditions in which RESET was blocked with BRD4780, we detected a sharp decrease in antibody-uptake after the 3-hour BRD4780-pretreatment of YFP-PrP* NRK cells (Figure 6A-B, Streptavidin-DyLight800-blot, compare lanes 6 and 8). Despite demonstrating the expected increase of antibody-uptake in TG-treated cells (Figure 6 A-B, Streptavidin-DyLight800-blot, compare lanes 6 and 8), the 3-hour BRD4780-pretreatment inhibited TG-induced antibody-uptake (Figure 6A-B, Streptavidin-DyLight800-blot, compare lanes 7 and 9). To quantify the effects of BRD4780 on antibody-uptake and YFP-PrP* levels, we performed the experiment in triplicate (Figure 6C-D). As shown previously (Figure 5C), anti-GFP antibody-uptake in YFP-PrP* NRK cells approximately doubled in the presence of TG “YFP-PrP* +Baf +Ab +TG” when compared to baseline-levels “YFP-PrP* +Baf +Ab” (Figure 6C). However, anti-GFP antibody-uptake was inhibited in cells with 3-hour BRD4780-pretreatment in the absence of TG “YFP-PrP* BRD4780 P-T +Baf +Ab” or the presence of TG “YFP-PrP* BRD4780 P-T +Baf +Ab +TG” (Figure 6C). Taken together, we have demonstrated the utility of our western blot-based approach to detect decreased antibody-uptake in response to a small molecule that inhibits traffic of an integral membrane protein-of-interest to the PM (Figure 6).
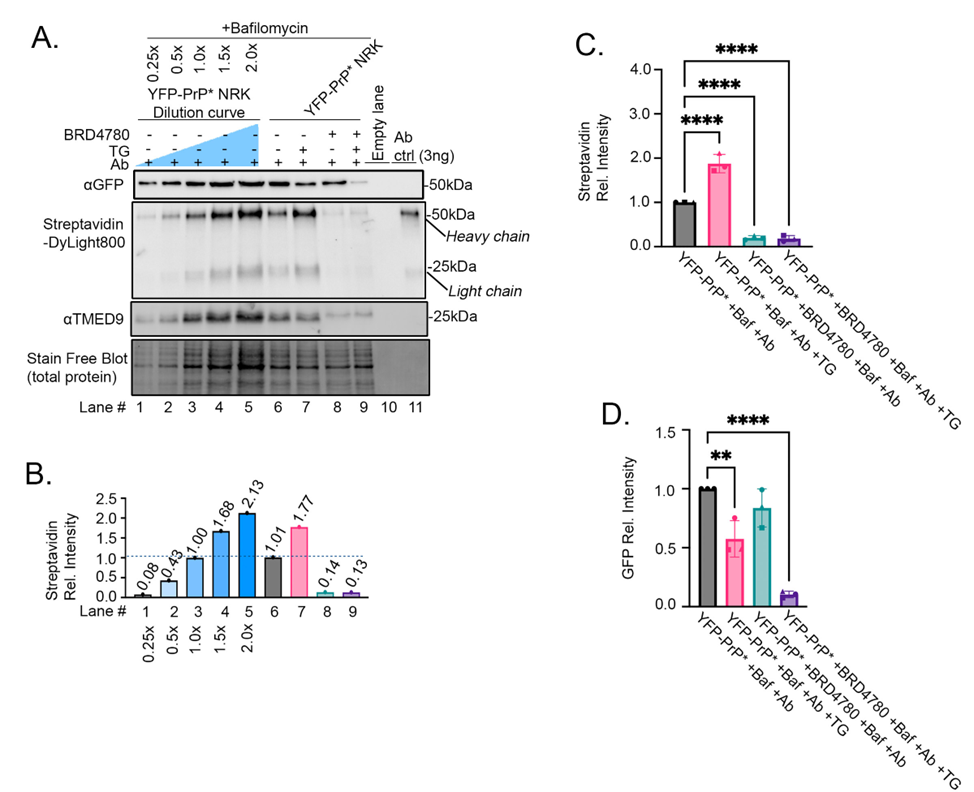
Figure 6: Proof-of-principle experiment demonstrating the expected decrease in anti-GFP antibody-uptake after BRD4780 treatment as detected by our western blot-based approach. (A) Western blot of YFP-PrP* NRK cell lysates from cells that were incubated for 2 h with 500 nM Bafilomycin A1, 1μg/mL antibody (Ab), 100μM BRD4780 and 0.1μM TG as indicated. For conditions with BRD4780 treatment, a 3-hour pretreatment was included prior to the 2-hour co-incubation with Bafilomycin, antibody and TG as indicated. Lanes 1-5 represent a dilution curve of the YFP-PrP* NRK cell lysates that were incubated for 2h with 500 nM Bafilomycin A1 and 1μg/mL antibody (Ab). The membrane was imaged for total protein using the Stain-Free™ system then probed with Streptavidin-DyLight800. A second membrane was probed with rabbit anti-GFP polyclonal antibody, followed by HRP-conjugated anti-rabbit secondary antibody, and with mouse anti-TMED9 antibody followed by a fluorescent anti-mouse secondary antibody. (B) Quantification of the Streptavidin-DyLight800-bound heavy chain bands of the biotinylated anti-GFP antibody. All of the band intensities were normalized against the 1X dilution of the YFP-PrP* NRK cell lysates. (C) Quantification of Streptavidin-DyLight800-bound heavy chain bands of the internalized biotinylated anti-GFP antibody. Data is presented as means and standard deviations (n=3). All of the band intensities were normalized against the total protein loaded in the stain free blot and also the 1X dilution of the YFP-PrP* NRK cell lysates. Symbols represent each of the 3 trials. The circles correspond to the blot shown in (A). Four asterisks indicate statistically significantly different from YFP-PrP* +Baf +Ab (p<0.0001) using an ordinary one-way ANOVA test comparing each group to the control condition (YFP-PrP* +Baf +Ab) with Dunnett’s correction for multiple comparisons. Means and standard deviations are as follows: YFP-PrP* +Baf +Ab, 1.000 +/- 0; YFP-PrP* +Baf +Ab +TG, 1.876 +/- 0.2080; YFP-PrP* +BRD4780 +Baf +Ab, 0.1989 +/- 0.4944; YFP-PrP* +BRD4780 +Baf +Ab +TG, 0.1826 +/- 0.06759. (D) Quantification of anti-GFP antibody detecting YFP-PrP* western blot bands. Data is presented as means and standard deviations (n=3). All of the band intensities were normalized against the total protein loaded in the stain free blot and also the 1X dilution of the YFP-PrP* NRK cell lysates. Symbols represent each of the 3 trials. The circles correspond to the blot shown in (A). Asterisks indicate statistically significantly different from YFP-PrP* +Baf +Ab (p<0.05) using an ordinary one-way ANOVA test comparing each group to the control condition (YFP-PrP* +Baf +Ab) with Dunnett’s correction for multiple comparisons. Four asterisks indicate p <0.0001, two asterisks indicate p<0.005. Means and standard deviations are as follows: YFP-PrP* +Baf +Ab, 1.000 +/- 0; YFP-PrP* +Baf +Ab +TG, 0.5745 +/- 0.1544; YFP-PrP* +BRD4780 +Baf +Ab, 0.8372 +/- 0.1618; YFP-PrP* +BRD4780 +Baf +Ab +TG, 0.1036 +/- 0.02773.
An exciting observation revealed by this proof-of-principle experiment was that TG-treatment in 3-hour BRD4780-pretreated cells induced rapid degradation of YFP-PrP*, despite co-incubation of TG with the lysosomal degradation inhibitor Bafilomycin A1 (Figure 6A-B, GFP-blot, compare lanes 6 and 9). Triplicate analysis and quantification of the GFP blots revealed that YFP-PrP* levels did not change significantly in the presence of the 3-hour BRD4780-pretreatment “YFP-PrP* BRD4780 P-T +Baf +Ab” when compared to baseline-levels “YFP-PrP* +Baf +Ab” (Figure 6D). However the combination of BRD4780 and TG “YFP-PrP* BRD4780 P-T +Baf +Ab +TG” induced a significant drop in the YFP-PrP* levels when compared to baseline-levels “YFP-PrP* +Baf +Ab” (Figure 6D). Thus, Figure 6 demonstrates that under conditions in which RESET is blocked, YFP-PrP* is degraded through an alternate pathway that (a) does not involve trafficking to the cell surface and (b) is not inhibited by Bafilomycin A1. The depletion in YFP-PrP* levels in the presence of lysosomal degradation inhibitor, Bafilomycin A1, suggests that this alternate pathway may not involve lysosomal degradation. Further studies will need to be performed in order to determine the mechanism underlying this new observation. Notably, the results in Figure 6 demonstrate that this new western blot-based antibody-uptake protocol facilitates new discoveries relating to protein trafficking pathways, degradation and homeostasis.
In summary, we have used YFP-PrP* as a model substrate to demonstrate how biotinylated antibody uptake can be used to monitor integral protein trafficking to and internalization from the PM (Figure 1, bold purple arrows). To validate our new western blot-based antibody-uptake protocol, we took advantage of previous findings that tagged variants of PrP* are efficiently endocytosed from the PM and that traffic to the PM can be pharmacologically modified (Figure 3 B-E). Using this western blot-based biotinylated-antibody-uptake assay, we replicated the expected findings that TG or BRD4780 respectively promote or inhibit YFP-PrP* trafficking to the PM. Interestingly, Figure 6 revealed that pretreatment with BRD4780 reroutes YFP-PrP* degradation through a pathway that does not involve trafficking to the cell surface and is not inhibited by Bafilomycin A1. While performing these protocol validation experiments, we demonstrated and exploited the following benefits of our western blot-based approach to detect antibody-uptake: (a) the accessibility of SDS-PAGE and western blot equipment and reagents, (b) the high binding affinity of streptavidin for biotinylated proteins and specificity surpassing that of traditional primary-secondary antibody pairs, (c) the relatively short training time for a new student to learn the entire workflow, (d) the short turnaround time from sample collection to quantified streptavidin bands, and (e) the semi-quantitative nature of western blots. Thus, we demonstrate here the facility of this protocol to make new discoveries relating to integral membrane protein trafficking pathways and degradation. This protocol may be easily adjusted to use with other adherent or non-adherent cell lines, primary or differentiated cell cultures to monitor antibody-uptake by any integral membrane protein.
General notes and troubleshooting
General notes
This protocol could be modified for use with non-adherent cell culture lines, differentiated cells or primary cell culture derived from animal tissues.
Potential limitations of this technique that must be taken into account include the following:
In some cases, antibody-binding to the integral membrane protein may alter the kinetics of endocytosis, either by crosslinking the integral membrane proteins or by interfering with their ligand binding [2,3,5,44].
This protocol is dependent on the availability and specificity of the biotinylated primary antibody to the antigen on the extracellular portion of the protein in the context of its native and folded form.
Troubleshooting
Problem 1: Specific antibody-uptake of biotinylated primary antibody was undetectable.
Possible cause: Biotinylated antibody has poor binding affinity to the integral membrane protein-of-interest. The antibody may not recognize the epitope in the native folded protein as it is presented in live cell culture, but instead only recognize the epitope in its denatured, unfolded form.
Solution: Try a different biotinylated antibody, preferably a polyclonal antibody raised against an antigen that was introduced into the animal in its native form. Before landing on the biotinylated goat polyclonal anti-GFP antibody (Genetex, GTX26658) as the reagent that we used in our above proof-of-principle experiments, we tested other antibodies. For example, we found that anti-GFP antibody-uptake did not work for Alpaca derived GFP VHH, biotinylated recombinant nanobody (Proteintech, gtb-250, used at 1:500 diluted in medium).
Problem 2: A high level of biotinylated antibody-uptake was detected in the negative control conditions.
Possible cause A: A low-level of pinocytosis results in non-specific antibody-uptake. We detect this in our negative controls (Figure 5C “NRK +Baf +Ab” and “YFP-CD3δ +Baf +Ab”), which display ~10-20% antibody-uptake levels of the base-line positive control (Figure 5C “YFP-PrP* +Baf +Ab”). Over time or with too high of a concentration of the biotinylated primary antibody in the medium, the non-specific antibody-uptake may mask the signal from specific antibody-uptake.
Solution A: Reduce either the incubation time or concentration of primary biotinylated antibody until you identify an incubation period or dilution where non-specific antibody-uptake can be differentiated from specific antibody-uptake.
Possible cause B: Biotinylated primary antibody binds to an alternate antigen that is displayed more dominantly on the cell surface than your protein-of-interest.
Solution B: Try different primary biotinylated antibodies until you find one where non-specific antibody-uptake can be differentiated from specific antibody-uptake.
Acknowledgments
Author Contributions: Investigation, A.M.G and P.S-K. Writing—Original Draft, A.M.G and P.S-K.; Writing—Review & Editing, A.M.G and P.S-K.; Conceptualization, Funding acquisition, and Supervision, P.S-K.
Funding sources: This research was supported by National Institute of General Medical Sciences National Institutes of Health grant no. R01 GM134327 and The Uniformed Services University of the Health Sciences (USU, 4301 Jones Bridge Rd., A1040C, Bethesda, MD 20814-4799) grant no. HU00012320103. This project is sponsored by USU; however, the information or content and conclusions do not necessarily represent the official position or policy of, nor should any official endorsement be inferred on the part of, USU, the Department of Defense, or the U.S. Government.
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
This work did not use human or animal subjects and therefore has no ethical considerations.
References
1. Tsaltas, G., Ford, C. H. and Gallant, M. (1992). Demonstration of monoclonal anti-carcinoembryonic antigen (CEA) antibody internalization by electron microscopy, western blotting and radioimmunoassay. Anticancer Res 12(6B): 2133-2142.
2. Garrigues, J., Garrigues, U., Hellstrom, I. and Hellstrom, K. E. (1993). Ley specific antibody with potent anti-tumor activity is internalized and degraded in lysosomes. Am J Pathol 142(2): 607-622.
3. Louvard, D. (1980). Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A 77(7): 4132-4136. https://doi.org/10.1073/pnas.77.7.4132.
4. Herz, J., Kowal, R. C., Ho, Y. K., Brown, M. S. and Goldstein, J. L. (1990). Low density lipoprotein receptor-related protein mediates endocytosis of monoclonal antibodies in cultured cells and rabbit liver. J Biol Chem 265(34): 21355-21362.
5. Beisiegel, U., Schneider, W. J., Goldstein, J. L., Anderson, R. G. and Brown, M. S. (1981). Monoclonal antibodies to the low density lipoprotein receptor as probes for study of receptor-mediated endocytosis and the genetics of familial hypercholesterolemia. J Biol Chem 256(22): 11923-11931.
6. Gottlieb, T. A., Ivanov, I. E., Adesnik, M. and Sabatini, D. D. (1993). Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol 120(3): 695-710. https://doi.org/10.1083/jcb.120.3.695.
7. Ford, C. H., Tsaltas, G. C., Osborne, P. A. and Addetia, K. (1996). Novel flow cytometric analysis of the progress and route of internalization of a monoclonal anti-carcinoembryonic antigen (CEA) antibody. Cytometry 23(3): 228-240. https://doi.org/ https://doi.org/10.1002/(SICI)1097-0320(19960301)23:3<228::AID-CYTO6>3.0.CO;2-E.
8. Liu, H., Rajasekaran, A. K., Moy, P., Xia, Y., Kim, S., Navarro, V., Rahmati, R. and Bander, N. H. (1998). Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res 58(18): 4055-4060.
9. Chivers, C. E., Koner, A. L., Lowe, E. D. and Howarth, M. (2011). How the biotin-streptavidin interaction was made even stronger: investigation via crystallography and a chimaeric tetramer. Biochem J 435(1): 55-63. https://doi.org/10.1042/BJ20101593.
10. Liu, F., Zhang, J. Z. and Mei, Y. (2016). The origin of the cooperativity in the streptavidin-biotin system: A computational investigation through molecular dynamics simulations. Sci Rep 6: 27190. https://doi.org/10.1038/srep27190.
11. Luong, J. H. T. and Vashist, S. K. (2020). Chemistry of Biotin-Streptavidin and the Growing Concern of an Emerging Biotin Interference in Clinical Immunoassays. ACS Omega 5(1): 10-18. https://doi.org/10.1021/acsomega.9b03013.
12. Green, N. M. (1990). Avidin and streptavidin. Methods Enzymol 184: 51-67. https://doi.org/10.1016/0076-6879(90)84259-j.
13. Laitinen, O. H., Hytonen, V. P., Nordlund, H. R. and Kulomaa, M. S. (2006). Genetically engineered avidins and streptavidins. Cell Mol Life Sci 63(24): 2992-3017. https://doi.org/10.1007/s00018-006-6288-z.
14. Liu, S., Zhang, H., Dai, J., Hu, S., Pino, I., Eichinger, D. J., Lyu, H. and Zhu, H. (2015). Characterization of monoclonal antibody's binding kinetics using oblique-incidence reflectivity difference approach. MAbs 7(1): 110-119. https://doi.org/10.4161/19420862.2014.985919.
15. Landry, J. P., Ke, Y., Yu, G. L. and Zhu, X. D. (2015). Measuring affinity constants of 1450 monoclonal antibodies to peptide targets with a microarray-based label-free assay platform. J Immunol Methods 417: 86-96. https://doi.org/10.1016/j.jim.2014.12.011.
16. Belitzky, E., Cavaliere, A., Rajabimoghadam, K. and Marquez-Nostra, B. (2022). Determining Binding Affinity (KD) of Radiolabeled Antibodies to Immobilized Antigens. J Vis Exp (184). https://doi.org/10.3791/63927.
17. Abcam Company (2025). KD value: a quantitative measurement of antibody affinity. Weblink: https://www.abcam.co.jp/primary-antibodies/kd-value-a-quantitive-measurement-of-antibody-affinity.
18. Ronzier, E. and Satpute-Krishnan, P. (2025). TMED9 coordinates the clearance of misfolded GPI-anchored proteins out of the ER and into the Golgi. PLoS Biol 23(4): e3003084. https://doi.org/10.1371/journal.pbio.3003084.
19. Cheatham, A. M., Sharma, N. R. and Satpute-Krishnan, P. (2023). Competition for calnexin binding regulates secretion and turnover of misfolded GPI-anchored proteins. J Cell Biol 222(10). https://doi.org/10.1083/jcb.202108160.
20. Satpute-Krishnan, P., Ajinkya, M., Bhat, S., Itakura, E., Hegde, R. S. and Lippincott-Schwartz, J. (2014). ER stress-induced clearance of misfolded GPI-anchored proteins via the secretory pathway. Cell 158(3): 522-533. https://doi.org/10.1016/j.cell.2014.06.026.
21. Zavodszky, E. and Hegde, R. S. (2019). Misfolded GPI-anchored proteins are escorted through the secretory pathway by ER-derived factors. Elife 8. https://doi.org/10.7554/eLife.46740.
22. Roberts, B. S., Mitra, D., Abishek, S., Beher, R. and Satpute-Krishnan, P. (2024). The p24-family and COPII subunit SEC24C facilitate the clearance of alpha1-antitrypsin Z from the endoplasmic reticulum to lysosomes. Mol Biol Cell 35(3): ar45. https://doi.org/10.1091/mbc.E23-06-0257.
23. Tsien, R. Y. (1998). The green fluorescent protein. Annu Rev Biochem 67: 509-544. https://doi.org/10.1146/annurev.biochem.67.1.509.
24. Hailey, D. W., Rambold, A. S., Satpute-Krishnan, P., Mitra, K., Sougrat, R., Kim, P. K. and Lippincott-Schwartz, J. (2010). Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141(4): 656-667. https://doi.org/10.1016/j.cell.2010.04.009.
25. Bonifacino, J. S., Suzuki, C. K., Lippincott-Schwartz, J., Weissman, A. M. and Klausner, R. D. (1989). Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J Cell Biol 109(1): 73-83. https://doi.org/10.1083/jcb.109.1.73.
26. Chen, C., Bonifacino, J. S., Yuan, L. C. and Klausner, R. D. (1988). Selective degradation of T cell antigen receptor chains retained in a pre-Golgi compartment. J Cell Biol 107(6 Pt 1): 2149-2161. https://doi.org/10.1083/jcb.107.6.2149.
27. Bowman, E. J., Siebers, A. and Altendorf, K. (1988). Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A 85(21): 7972-7976. https://doi.org/10.1073/pnas.85.21.7972.
28. Dvela-Levitt, M., Kost-Alimova, M., Emani, M., Kohnert, E., Thompson, R., Sidhom, E. H., Rivadeneira, A., Sahakian, N., Roignot, J., Papagregoriou, G., et al. (2019). Small Molecule Targets TMED9 and Promotes Lysosomal Degradation to Reverse Proteinopathy. Cell 178(3): 521-535 e523. https://doi.org/10.1016/j.cell.2019.07.002.
29. Daoud Sarsour, A., Kinstlinger, S., Nizar, R., Amos, N., Arbeli, N., Kazimirsky, G., Bronshtein-Berger, I., Fried, I., Unger, R., Brodie, C., et al. (2025). Targeting the Cargo Receptor TMED9 as a Therapeutic Strategy Against Brain Tumors. Cells 14(11). https://doi.org/10.3390/cells14110772.
30. Vredevoogd, D. W., Apriamashvili, G., Levy, P. L., Sinha, S., Huinen, Z. R., Visser, N. L., de Bruijn, B., Boshuizen, J., van Hal-van Veen, S. E., Ligtenberg, M. A., et al. (2024). TMED inhibition suppresses cell surface PD-1 expression and overcomes T cell dysfunction. J Immunother Cancer 12(11). https://doi.org/10.1136/jitc-2024-010145.
31. Bazua-Valenti, S., Brown, M. R., Zavras, J., Riedl Khursigara, M., Grinkevich, E., Sidhom, E. H., Keller, K. H., Racette, M., Dvela-Levitt, M., Quintanova, C., et al. (2024). Disrupted uromodulin trafficking is rescued by targeting TMED cargo receptors. J Clin Invest 134(24). https://doi.org/10.1172/JCI180347.
32. Lorenz, H., Hailey, D. W. and Lippincott-Schwartz, J. (2006). Fluorescence protease protection of GFP chimeras to reveal protein topology and subcellular localization. Nat Methods 3(3): 205-210. https://doi.org/10.1038/nmeth857.
33. Wileman, T., Kane, L. P., Carson, G. R. and Terhorst, C. (1991). Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J Biol Chem 266(7): 4500-4507.
34. Wileman, T., Pettey, C. and Terhorst, C. (1990). Recognition for degradation in the endoplasmic reticulum and lysosomes prevents the transport of single TCR beta and CD3 delta subunits of the T-cell antigen receptor to the surface of cells. Int Immunol 2(8): 743-754. https://doi.org/10.1093/intimm/2.8.743.
35. Strating, J. R. and Martens, G. J. (2009). The p24 family and selective transport processes at the ER-Golgi interface. Biol Cell 101(9): 495-509. https://doi.org/10.1042/BC20080233.
36. Pastor-Cantizano, N., Montesinos, J. C., Bernat-Silvestre, C., Marcote, M. J. and Aniento, F. (2016). p24 family proteins: key players in the regulation of trafficking along the secretory pathway. Protoplasma 253(4): 967-985. https://doi.org/10.1007/s00709-015-0858-6.
37. Roberts, B. S. and Satpute-Krishnan, P. (2022). The many hats of transmembrane emp24 domain protein TMED9 in secretory pathway homeostasis. Front Cell Dev Biol 10: 1096899. https://doi.org/10.3389/fcell.2022.1096899.
38. Wada, I., Rindress, D., Cameron, P. H., Ou, W. J., Doherty, J. J., 2nd, Louvard, D., Bell, A. W., Dignard, D., Thomas, D. Y. and Bergeron, J. J. (1991). SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem 266(29): 19599-19610.
39. Ahluwalia, N., Bergeron, J. J., Wada, I., Degen, E. and Williams, D. B. (1992). The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J Biol Chem 267(15): 10914-10918.
40. Degen, E. and Williams, D. B. (1991). Participation of a novel 88-kD protein in the biogenesis of murine class I histocompatibility molecules. J Cell Biol 112(6): 1099-1115. https://doi.org/10.1083/jcb.112.6.1099.
41. David, V., Hochstenbach, F., Rajagopalan, S. and Brenner, M. B. (1993). Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin). J Biol Chem 268(13): 9585-9592.
42. Schlatter, S., Stansfield, S. H., Dinnis, D. M., Racher, A. J., Birch, J. R. and James, D. C. (2005). On the optimal ratio of heavy to light chain genes for efficient recombinant antibody production by CHO cells. Biotechnol Prog 21(1): 122-133. https://doi.org/10.1021/bp049780w.
43. Murphy, K., Travers, P., Walport, M. and Janeway, C. (2012). Janeway's immunobiology. Garland Science. New York. ISBN: 97808153424340815342438.
44. Paul, D., Stern, O., Vallis, Y., Dhillon, J., Buchanan, A. and McMahon, H. (2023). Cell surface protein aggregation triggers endocytosis to maintain plasma membrane proteostasis. Nat Commun 14(1): 947. https://doi.org/10.1038/s41467-023-36496-y.
- Graninger, A and Satpute-Krishnan, P(2025). Monitoring endocytosis of integral membrane proteins using western blot-based detection of biotinylated antibody-uptake. Bio-protocol Preprint. DOI: 10.21769/p2855.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link
