Advanced Search
Purification of endogenous lysyl oxidase (LOX) from MEF cell culture
Last updated date: Nov 28, 2024 Views: 99 Forks: 0
Abstract
Lysyl Oxidase (LOX) is a copper-dependent enzyme that oxidizes primary amine in peptidyl lysine residues to reactive aldehydes, thereby mediates cross-linking of collagens and elastin. Purification of LOX from skin or aorta tissues were described elsewhere, but we successfully purified LOX from murine embryonic fibroblast (MEF) culture that could be used for enzyme activity assay, N-terminal amino acid sequencing, and mass-spectrometric analysis. Using this method, we showed that fibulin-4 is required for the activation of LOX. Here we describe the protocol for purification of LOX from MEF culture.
Materials and Reagents
MEF cell line that highly express LOX (described below)
Advanced DMEM (Thermo, Cat. 12491015)
Advanced DMEM/F12 (Termo, Cat. 12634028)
Penicillin-Streptomycin-Glutamine (100X) (Thermo, Cat. 10378016)
Trypsine-EDTA (0.25%), phenol red (Thermo, Cat. 25200072)
Fetal Bovine Serum (Biosera)
15 cm cell culture plate (Thermo, Cat. 130183)
DEAE Sepharose Fast Flow resin (Cytiva, Cat. 17070901, formerly GE healthcare)
Sephacryl S-200 HR (Cytiva, Cat. 17058401, formerly GE healthcare)
Empty Column XK 26/20 (Cytiva, Cat. 28988948, formerly GE healthcare)
anti-LOX antibody (rabbit, polyclonal) (abcam, Cat. ab31238)
Can Get Signal Solution (Immunoreaction Enhancer) (Toyobo, Cat. NKB-101)
Blocking One (Blocking solution for Western blot) (Nacalai Tesque, Cat. 28988949)
Equipment
Medium-pressure chromatography system (we used Bio-Rad BioLogic Duoflow system, but any system of medium to high pressure (HPLC) should work)
XCell SureLock Mini-Cell electrophoresis system (Thermo, Cat. EI0002)
Snap i.d. 2.0 Protein Detection System (for quick Western blot) (Merck)
Procedure
Prepare MEF cells immortalized by infecting TERT lentivirus. MEF cells may be spontaneously immortalized without TERT overexpression.
Isolate 20 - 24 MEF cell lines by limiting dilution (dilute cell suspension and seed them in 96-well plates at 0.5 cell/well). Freeze-stock the cell lines.
Select a MEF cell line that secrete LOX at the highest level. This procedure is most important because LOX expression levels largely differ among the cell lines.
Culture the MEF cell lines in 6-well plates until confluent with Advanced DMEM, 1x Penicillin-Streptomycin-Glutamine, 5% FBS (hereafter "growth medium").
Change the medium with 1.5 mL of Advanced DMEM/F12, 1x Penicillin-Streptomycin-Glutamine (serum-free or 0.5% FBS) (hereafter "harvest medium"). Culture in 5% CO2 for 2 - 3 days.
Harvest the conditioned media (CM), spin down to remove dead cell debris, and transfer the sup to new tubes.
Add DEAE Sepharose FF slurry 20 µL to the CM. Rotate at 4 ºC for 1 hr.
Spin down and aspirate the sup (LOX is bound to the DEAE Sepharose FF).
Add PBS 25 µL, 10x NuPAGE Sample Reducing Agent (Thermo) 6 µL, 4x LDS Sample Buffer (Thremo) 15 µL, and heat at 75 ºC, 10 min.
Spin down and transfer the sup to new tubes. Load 10 µL/lane in NuPAGE 4 - 12% SDS-PAGE Bis-Tris gel (Thermo) and run in MES buffer (Thermo). Transfer to a PVDF membrane pre-activated with methanol.
Perform Western blot according to the manufacturer's manual for Snap i.d. 2.0 (Merck). Blocking: Blocking One (Nacalai Tesque) diluted by 1/5 with Tris-buffered saline (TBS). Primary Ab: anti-LOX polyclonal Ab (abcam) diluted by 1/2000 with Can Get Signal Solution 1 (Toyobo). Secondary Ab: HRP-anti-rabbit IgG (Santa Cruz) diluted by 1/2000 with Can Get Signal Solution 2 (Toyobo). Chemiluminescent solution: Immunostar LD (Fujifilm).
Expand the MEF cell line that highly express LOX with growth medium to confluent 15 cm plate x 24.
Change the medium with harvest medium 16 mL/plate. Culture for 2 - 3 days.
Harvest CM to 50 mL conical tubes x 8. Centrifuge at 3 krpm for 5 min and transfer the sup to new tubes.
First step purification with DEAE Sepharose FF
Add DEAE Sepharose FF slurry 1 mL/tube and rotate at 4 ºC for 2 hr. LOX attaches to the DEAE Sepharose FF.
Centrifuge at 3 krpm for 5 min and discard the sup. Pack an empty column (a 20 mL syringe with cotton stuffed in the tip) with the recovered DEAE Spharose FF resin.
Attach a tube at the column outflow and aspirate with a peristaltic pump, while adding following buffers on top of the column to wash the resin.
Phosphate buffer (10 mM, pH 7.8) (PB) 20 mL
PB-NaCl (10 mM PB, 1 M NaCl) 20 mL (surprisingly, LOX is not eluted with this)
PB 20 mL
PBU (10 mM PB, 6 M Ultra-pure Urea) 20 mL
Remove the tube from the column and elute LOX by adding PBU-NaCl (10 mM PB, 6 M Ultra-pure Urea, 1 M NaCl) 20 mL onto the column. We discarded the initial 3 mL and collect the following 10 mL as the eluate, but this should be empirically determined by Western blot of each fraction.
Dialyze the eluate (10 mL) against PB (1 L x 2 times) with Slide-A-Lyzer 10k (Thermo) at 4 ºC overnight.
Second step purification with Sephacryl S-200HR
Pack a XK26/20 column with 25 mL of Sephacryl S-200HR. Set the column to a chromatography system. The system should not be refrigerated.
Pump A: PB (10 mM, pH7.8), Pump B: PBU (10 mM PB, 6M Ultra-pure Urea).
PB (pump A 100%) 100 mL, 5 mL/min, then pause
Sample injection (dialyzed sample 10 mL) either with loop or manually with syringe
PB (pump A 100%) 100 mL, 5 mL/min
Start fraction collector (2.5 mL/fraction), PBU (pump B 100%) 50 mL, 3 mL/min. LOX were eluted at fraction 11 - 14 in our system, but this should be empirically determined by Western blot of each fraction.
PBU (pump B 100%) 50 mL, 5 mL/min to wash the column
Concentrate the LOX-containing fractions to 100 - 200 µL with Amicon Ultra 10k (Merck).
Check the concentration and purity of the LOX using SDS-PAGE and CBB staining.
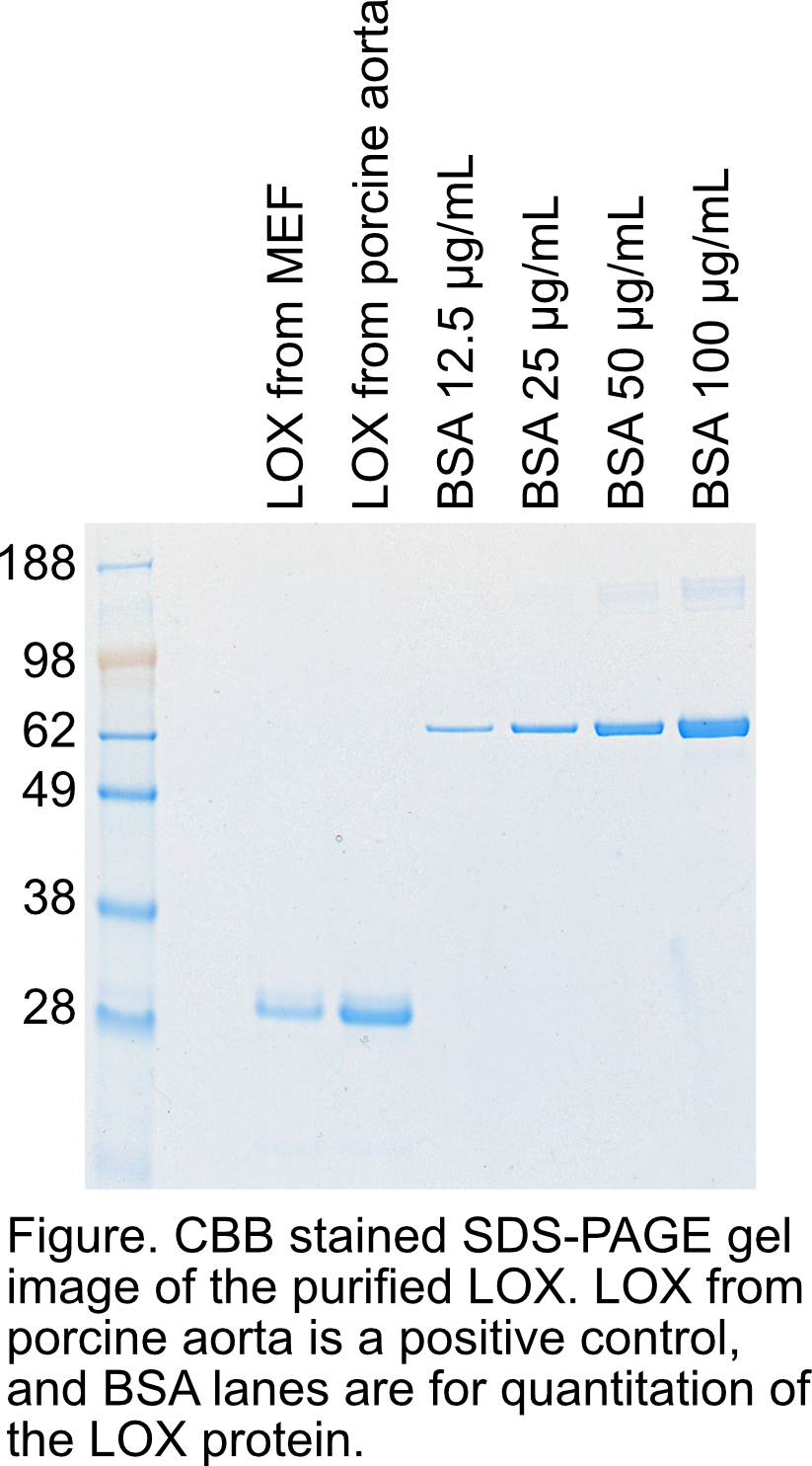
Controls
We use LOX purified from pig aorta as a positive control. Three to five pig aortae are minced and proteins are extracted overnight with PBU. As LOX binds with DEAE Sepharose FF in PBU, LOX can be purified using sequential purification with DEAE Sepharose FF and Sephacryl S-200HR as shown above. MEF cell line that overexpress full-length LOX cDNA by lentivirus may also be a positive control. However, in our experiments, MEF cell lines selected for high endogenous LOX expression secrete as much LOX as LOX-overexpressing lines. 293T cells overexpressing full-length LOX cDNA can also be used as a control for purification procedure, but caution is needed because LOX secreted from 293T does not have LTQ, which is shown by slightly delayed migration in SDS-PAGE, and therefore is enzymatically inactive.
Copyright: Content may be subjected to copyright.
How to cite:
Readers should cite both the Bio-protocol preprint and the original research article where this protocol was used:
1. Noda K, Kitagawa K, Miki T, Horiguchi M, Akama TO, Taniguchi T, Taniguchi H, Takahashi K, Ogra Y, Mecham RP, Terajima M, Yamauchi Y, Nakamura T: A matricellular protein fibulin-4 is essential for the activation of lysyl oxidase. Sci Adv 6(48):eabc1404, 2020. doi: 10.1126/sciadv.abc1404.
- Nakamura, T(2024). Purification of endogenous lysyl oxidase (LOX) from MEF cell culture. Bio-protocol Preprint. bio-protocol.org/prep2763.
- Noda, K., Kitagawa, K., Miki, T., Horiguchi, M., Akama, T. O., Taniguchi, T., Taniguchi, H., Takahashi, K., Ogra, Y., Mecham, R. P., Terajima, M., Yamauchi, M. and Nakamura, T.(2020). A matricellular protein fibulin-4 is essential for the activation of lysyl oxidase . Science Advances 6(48). DOI: 10.1126/sciadv.abc1404
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
