Advanced Search
Environmental Scanning Electron Microscopy (SEM)
Last updated date: Oct 14, 2021 Views: 995 Forks: 0
Fast and high-resolution imaging of pollinated stigmatic cells by Tabletop Scanning Electron Microscopy
Abstract
In plants, the first interaction that occurs between the pollen grain and the epidermal cells of the stigma is crucial for successful reproduction. When accepted, the pollen germinates and emits a pollen tube that conveys the two sperm cells to the ovules where fertilization takes place. Confocal microscopy has been used to characterize the behavior of stigmatic cells following pollination (Rozier et al., 2020) but here we propose a fast and high resolution imaging protocol using a tabletop SEM. This technique, used in Riglet et al. (2020), does not require prior sample fixation or fluorescent marker lines. It allows the analysis of pollen grain behavior, from the very early hydration step (a few minutes after pollination) to the growth of the pollen tube within the stigma (an hour after pollination).
Materials and Equipment
- Tabletop SEM Hirox SH-3000
- Arabidopsis thaliana plants with flowers at stage 12 to 15 (Smyth et al., 1990)
- Stereomicroscope
- Fine tweezers
- Scalpel
- Scissors
Experimental procedure
A. Pollination procedure
Place inflorescences of a plant in its pot under the stereomicroscope. With fine tweezers, open flower buds from stage 12 (Smyth et al., 1990) and emasculate by carefully removing the anthers. Check the absence of pollen grains on stigma papillae (Figure 1A). Do not emasculate more than five buds on the same stem to avoid pistil dehydration.
Put the plants back to the culture room until the emasculated flowers have reached the desired developmental stages for pollination (see Smyth et al., 1990 for stages). For instance, to image stage 13 stigmas, emasculate flower buds at the end of stage 12 (Smyth et al., 1990) and wait 18 hours,
Hand pollinate under the stereomicroscope each emasculated flower still on the plant with mature wild-type pollen grains (e.g., flowers at stage 13) by carefully brushing the stigma with a dehiscent anther, so as to deposit only a small amount of pollen grains (as shown in Figure 1B). This allows the observation of both pollen grains and stigmatic cells during imaging,
Leave plants bearing pollinated flowers at room temperature on the bench. To image pollen hydration or foot appearance, wait for 10 to 15 minutes after pollination before proceeding to the imaging step, or 30 min to one hour to image long-growing pollen tubes,
Cut pollinated pistils with a scalpel in the middle of the ovary, transversally to their length, at the appropriate time after pollination (Figure 1C). Sample height should not exceed 0.5 cm.
B. Imaging set up procedure
Gently deposit several cut pistils, vertically on a double-sided tape placed on the SEM platform (Figure 1D)
Place the platform in the Hirox SEM SH-3000 (Figure 1E), making sure that the door of the SEM is well sealed,
- Select temperature: -20°C, and wait until the temperature is reached,
- Select accelerating voltage: 15kV,
- Turn on vaccum
- Imaging
C. Imaging
Observe samples within the first 15 minutes to prevent cell collapsing while imaging. To obtain a sharp image of the stigma surface, first zoom in at high magnification on pollen grains located in the middle of the stigma, and adjust the focus, contrast and brightness. Then, zoom out to have a broad view of the pistil.
Acquire the pictures (Figure 1F, G, H)
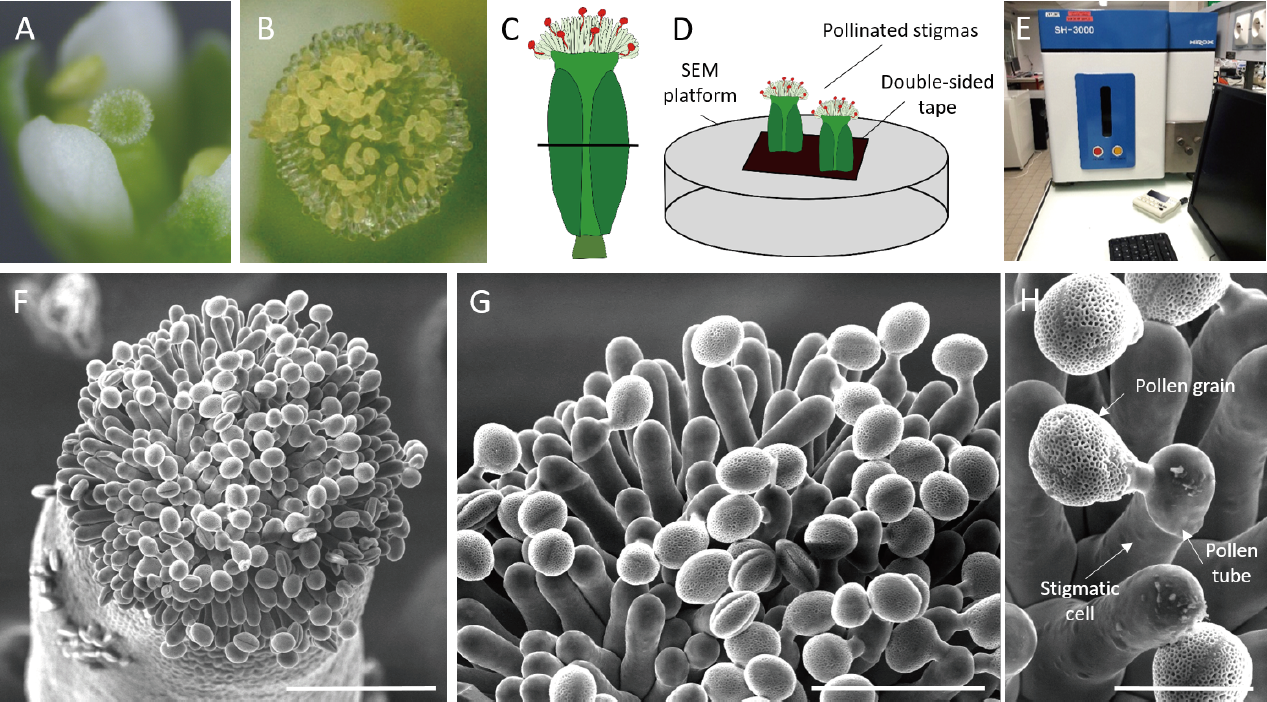
Figure 1: Imaging of pollinated Arabidopsis stigma using Scanning Electron Microscopy
(A) Stigma at stage 12, without pollen grains and before emasculation. (B) Stigma, a few minutes after hand-pollination. (C-D) Schematic view of (C) the position of the transversal cut of the pistil in the middle of the ovary, (D) the SEM set-up. (E) TableTop Hirox SEM SH-3000. (F-H) Top view of a pollinated stigma observed at (F) x300 magnification. Scale bar, 100 µm. (G) x700 magnification. Scale bar, 50 µm. (H) x 2000 magnification. Scale bar, 20 µm.
Acknowledgments
This protocol was originally described in and adapted from Riglet et al. (2020). This work was supported by a fellowship from the French Ministry of Higher Education and Research and by Grant ANR-14-CE11-0021.
References
Riglet L, Rozier F, Kodera C, Bovio S, Sechet J, Fobis-Loisy I, Gaude T. 2020. KATANIN- dependent mechanical properties of the stigmatic cell wall mediate the pollen tube path in Arabidopsis. eLife 9:e57282. doi:10.7554/eLife.57282
Rozier F, Riglet L, Kodera C, Bayle V, Durand E, Schnabel J, Gaude T, Fobis-Loisy I. 2020. Live- cell imaging of early events following pollen perception in self-incompatible Arabidopsis thaliana. Journal of Experimental Botany 71:2513–2526. doi:10.1093/jxb/eraa008
Smyth DR, Bowman JL, Meyerowitz EM. 1990. Early flower development in Arabidopsis. The Plant Cell 2:755–767. doi:10.1105/tpc.2.8.755
- Riglet, L, Fobis-Loisy, I and Gaude, T(2021). Environmental Scanning Electron Microscopy (SEM). Bio-protocol Preprint. bio-protocol.org/prep1408.
- Riglet, L., Rozier, F., Kodera, C., Bovio, S., Sechet, J., Fobis-Loisy, I. and Gaude, T.(2020). KATANIN-dependent mechanical properties of the stigmatic cell wall mediate the pollen tube path in Arabidopsis. eLife. DOI: 10.7554/eLife.57282
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

