- Home
- Protocols
-
Third generation lentivirus production and harvest by ultracentrifugation
 Timing: 1 week
Timing: 1 week
These steps describe how to generate 3rd generation LVs and has been adapted with some modifications from Hammill et al. (2016).
Resuspend 1.5–2 million HEK293T cells in 20 mL of complete DMEM media and transfer to a T-150 tissue culture flask. Culture flasks at 37°C and 5% CO2.
Note: Always start lentivirus prep with freshly thawed HEK293T cells to ensure higher viral yield. Around 2–3 80% confluent T-150 flasks are needed for one batch of lentiviral prep.
24 h prior to transfection, harvest HEK293T cells by washing flasks with 10 mL warm PBS and adding 3 mL warm 1× Trypsin-EDTA(0.05%). Place flasks in the incubator for 2–5 min and neutralize trypsin with 3× the volume of warm complete DMEM media.
Note: Cells should be ∼80% confluent three days post-thaw. HEK293T cells are very loosely adherent thus do not require a long incubation time with Trypsin. Use Trypsin to harvest cells to prevent cell clumping.
Transfer cell suspension to a 50 mL falcon tube and centrifuge at 300 × g for 5 min. Resuspend cell pellet with warm complete DMEM media and count cells using Trypan Blue.
Note: Use antibiotic-free complete DMEM media to plate cells prior to transfection as it may interfere with Lipofectamine 2000 reagent.
Note: Typically, three 15 cm plates are required to obtain sufficient viral particles per prep, scale up as needed. Carefully place plates into incubator ensuring that plates are even to make sure media covers each plate evenly.
Transfection can begin 24 h after plating HEK293T cells, at this point cells should be ∼70%–80% confluent.
Note: Measure concentration of all plasmids used prior to transfection using a spectrophotometer that measures the concentration and purity of nucleic acids.
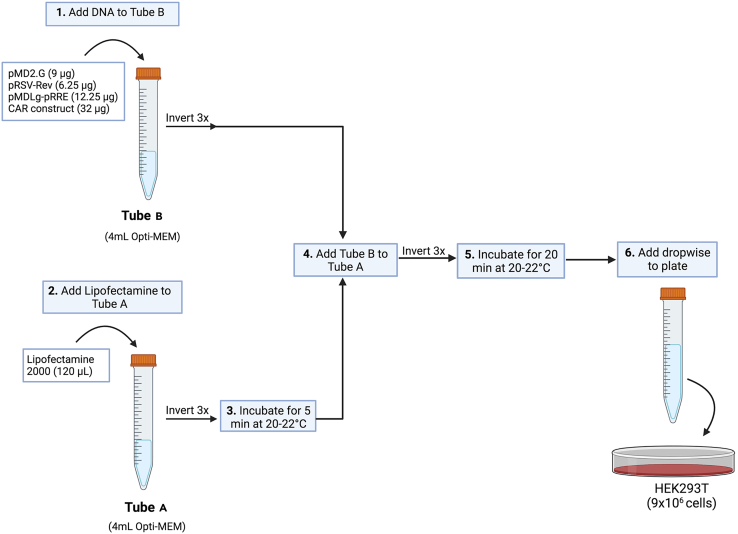
For each plate, prepare two 15 mL falcon tubes with 4 mL of Opti-MEM media labeled Tube A and Tube B.
Prepare transfection mixture by following schematic in Figure 2.
Slowly add the DNA and Lipofectamine mixture to each plate drop wise making sure to cover the entire plate area. Swirl plate carefully and slowly to evenly distribute the mixture.
Incubate plates at 37°C and 5% CO2.
Alternatives: Lipofecatamine (lipid based transfection agent) can be substituted with chemical methods such as calcium phosphate for the transfection. However, the amount of total DNA added should be optimized using this method. Refer to Brown et al. (2020) and Follenzi and Naldini (2002) for protocols on LV production using calcium phosphate.
12–16 h after transfection, carefully aspirate media and replace with 15 mL warm antibiotic-containing complete DMEM media supplemented with 1 mM of sodium butyrate.
Note: Sodium butyrate is added as it has been shown to enhance HIV-1 derived vector production (Olsen and Sechelski, 1995).
 CRITICAL: During this step, there may be lift off the HEK293T cells from the plate which can decrease viral yield. To avoid this ensure that DMEM media is warmed up to 37°C and aspirate media as slowly as possible. Only work with up to three plates at a time.
CRITICAL: During this step, there may be lift off the HEK293T cells from the plate which can decrease viral yield. To avoid this ensure that DMEM media is warmed up to 37°C and aspirate media as slowly as possible. Only work with up to three plates at a time.
Incubate plates at 37°C and 5% CO2.
Note: From this point forward, you will be working with virus samples. Ensure you are following proper BSL-2 guidelines when handling lentivirus.
36–48 h after transfection, lentivirus released in the supernatant can be harvested for viral concentration.
 CRITICAL: In our experience, the use of ultracentrifugation to concentrate the virus cannot be replaced with other methods of viral concentration such ultrafiltration. We have found that viral preps obtained from ultrafiltration do not efficiently transduce NK cells.
CRITICAL: In our experience, the use of ultracentrifugation to concentrate the virus cannot be replaced with other methods of viral concentration such ultrafiltration. We have found that viral preps obtained from ultrafiltration do not efficiently transduce NK cells.
Note: Pre-cool the SW-32 Ti ultracentrifuge rotor and buckets at 4°C before starting the harvest.
Aspirate viral containing supernatant from the 15 cm plates and transfer to 50 mL falcon tubes.
Centrifuge the 50 mL falcon tubes at 2000 × g for 10 min at 4°C to sediment any collected cells.
Filter supernatant using a 150 mL 0.45 μM PES filter to remove cell debris.
Alternatives: A filter with a pore size of 0.22 μM can also be used to improve purity. However, a size of 0.45 μM is used in this protocol to avoid any loss of viral particles during the filtration.
Add filtrate into 38.5 mL ultracentrifuge tubes and use cold PBS to bring tube within 0.5 cm of the edge to prevent centrifuge tube from collapsing.
Carefully balance the centrifuge tubes before placing into the ultracentrifuge buckets.
 CRITICAL: Ultracentrifuge buckets containing tubes must be balanced within 0.02g.
CRITICAL: Ultracentrifuge buckets containing tubes must be balanced within 0.02g.
Centrifuge buckets at 130,000 × g for 1 h 40 min at 4°C with minimal deceleration.
Carefully remove ultracentrifuge tube from ultracentrifuge bucket and pour the supernatant into a 20% bleach bucket.
Keep the ultracentrifuge tube inverted and blot the excess supernatant onto paper towels for up to 1 min to prevent viral pellet from drying out.
Immediately place the ultracentrifuge tube on ice and let it sit for 10 min to begin viral resuspension.
 CRITICAL: Avoid the formation of bubbles during resuspension of the viral pellet. Keep virus on ice at all times until placing in the −80°C freezer.
CRITICAL: Avoid the formation of bubbles during resuspension of the viral pellet. Keep virus on ice at all times until placing in the −80°C freezer.
Note: From this point forward only use micropipette filter tips when handling the virus.
Add between 50–100 μL of cold PBS and start to slowly scrape the bottom of the ultracentrifuge tube using a micropipette filter tip.
Note: Set the micropipette to a low volume (∼40 μL) and do not go to the second stop while resuspending to avoid formation of bubbles.
Note: The volume of PBS added to resuspend the viral pellet can be adjusted depending on your experience with the particular LV. For example, you can add a higher volume of PBS to lentivirus preps that have higher yields. If you consistently obtain a low viral yield see Troubleshooting 3.
Using the same micropipette filter tip, slowing resuspend the pellet by gently pipetting up and down and wash the sides of the ultracentrifuge tube.
Decontaminate anything that has come into contact with the virus using 70% ethanol.
Note: Minimize the production of aerosols when working in BSL2 conditions.
Flowchart depicting how to prepare transfection mixture
(1) Add required amount of plasmid DNA to Tube B and mix gently by inverting tube 3 times (3×). (2) Add 120 uL of Lipofectamine 2000 to Tube A and mix gently by inverting tube 3 times. (3) Incubate Tube A for 5 min at 20°C–22°C. (4) Add Tube B to Tube A, mix gently by inverting tube 3 times. (5) Incubate for 20 min at 20°C–22°C. (6) Add transfection mixture to each plate.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.