- EN - English
- CN - 中文
In Ovo CAM-Based Xenograft Model for Investigating Tumor Developmental Biology in Breast Cancer
基于鸡胚尿囊膜的体内异种移植模型用于研究乳腺癌肿瘤发育生物学
发布: 2026年02月20日第16卷第4期 DOI: 10.21769/BioProtoc.5600 浏览次数: 60
评审: Anonymous reviewer(s)
Abstract
Breast cancer remains one of the most prevalent and deadly malignancies affecting women worldwide. Its progression and metastatic behavior are driven by complex mechanisms. To develop more effective therapeutic strategies, it is crucial to understand tumor growth, angiogenesis, and microenvironmental interactions. Although traditional in vivo models such as murine xenografts have long been used to study tumor biology, these approaches are often time-consuming, costly, and ethically constrained. In contrast, the chick embryo chorioallantoic membrane (CAM) assay offers a rapid, cost-effective, and ethically flexible alternative for evaluating tumor development and angiogenesis. This protocol describes an in ovo CAM-based xenograft model in which human breast cancer cells are implanted onto the vascularized CAM of chick embryos. This method enables real-time evaluation of tumor growth. Furthermore, the model allows for manipulation of experimental conditions, including pharmacological treatments or genetic modifications, to study specific molecular mechanisms involved in breast cancer progression. The major advantages of this protocol lie in its simplicity, reduced cost, and capacity for high-throughput screening, making it a valuable tool for translational cancer research.
Key features
• Enables rapid tumor formation (3–4 days) after implantation of breast cancer cells.
• Ethical and low-cost alternative to rodent xenograft models; suitable for laboratories without animal facility infrastructure and aligned with the 3Rs principles.
• Optimized for short-term studies of tumor development, angiogenesis, and early metastatic events.
• Highly suitable for pharmacological testing and experimental manipulation of the tumor microenvironment.
Keywords: Breast cancer (乳腺癌)Graphical overview
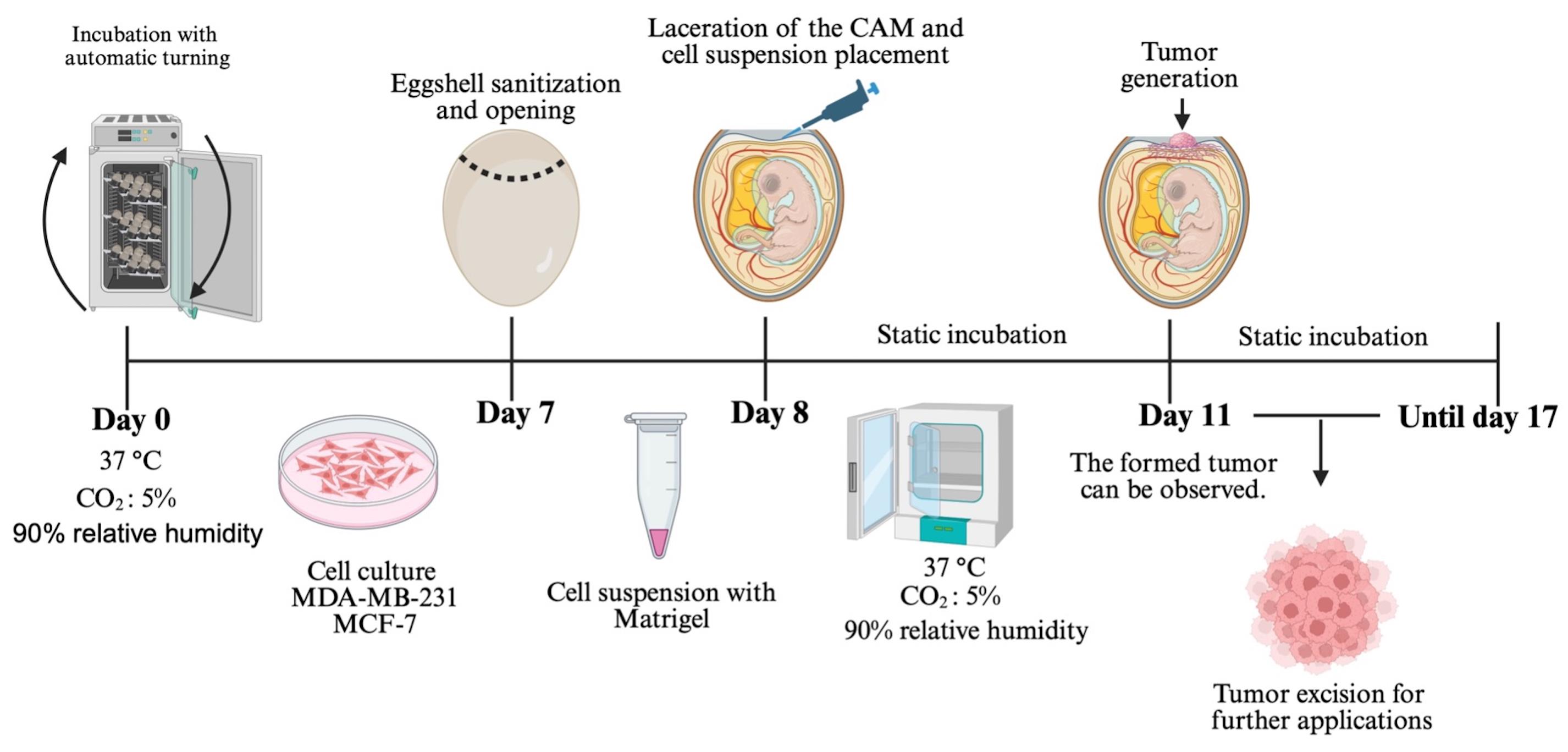
Chorioallantoic membrane (CAM) xenograft protocol. Fertilized eggs are incubated under automatic turning conditions until day 7, when eggshell windowing is performed. On day 8, the cell–Matrigel suspension is placed onto the exposed CAM. Embryos are maintained under static incubation, and tumor formation becomes visible from day 11. Tumors are collected for downstream applications.
Background
Breast cancer remains a significant global health concern. It is the most commonly diagnosed cancer in women and a leading cause of cancer-related deaths worldwide [1,2]. Epidemiological data indicate a continuous rise in incidence, largely associated with lifestyle factors, aging populations, and increased screening [3]. Despite substantial progress in clinical management driven by molecular subtyping, targeted therapies, immunotherapy, and improvements in early detection in recent decades, the clinical outcome for many patients continues to be negatively influenced by high tumor heterogeneity and frequent therapeutic resistance acquisition [4]. These characteristics limit the long-term success of current treatments and highlight the necessity of experimental approaches that allow a more precise understanding of breast cancer biology [2]. To achieve this, research models must not only replicate tumor-intrinsic molecular features but also key components of the tumor microenvironment, including stromal interactions, extracellular matrix composition, angiogenic processes, and the dynamic exchange of soluble factors [5]. Conventional two-dimensional (2D) cell culture systems have played an essential role in elucidating molecular pathways associated with proliferation, migration, and survival; however, they fail to reproduce the architectural and biochemical complexity of tumors in vivo [6]. Recently, three-dimensional (3D) culture platforms, including spheroids, scaffolds, organoids, and bioprinted constructs, have improved the modeling of cell–cell and cell–matrix interactions. However, limitations still exist when using these platforms to study angiogenesis, invasion, immune interactions, and metastatic potential [7]. For this reason, animal models are often necessary to validate in vitro observations and evaluate therapeutic responses in a context that is physiologically relevant [8].
Among animal systems, murine xenograft models have historically been the gold standard in preclinical breast cancer research. They permit long-term tumor development and allow evaluation of metastasis, immune modulation, and therapeutic efficacy. Nonetheless, their routine use poses significant challenges [9]. Rodent studies require specialized facilities, rigorous ethical reviews, significant financial resources, and extended timeframes for experimentation. These challenges, coupled with the growing emphasis on reducing, refining, and replacing animal experimentation (the 3Rs), have driven the development of alternative models that minimize animal suffering while maintaining biological relevance [10,11].
In this context, the chorioallantoic membrane (CAM) of the chick embryo has emerged as a powerful, versatile, and ethically advantageous platform for in vivo cancer research [12]. The CAM is a highly vascularized extraembryonic membrane that supports rapid tumor engraftment and neovascularization [13,14]. Importantly, the chicken embryo lacks a fully developed immune system during early incubation, which allows implantation of human cancer cells without the need for immunosuppression [15,16]. This cost-effective model requires minimal infrastructure and enables experimental timelines that are significantly shorter than those observed in rodents. Additionally, the CAM is not innervated during the early stages of development, which minimizes potential pain and reduces ethical concerns [17–21].
The present protocol was developed to provide a detailed, standardized, and reproducible methodology for inducing tumors on the CAM using human breast cancer cell lines. While the model has been successfully applied to melanoma, glioblastoma, colorectal, and head and neck cancers, breast cancer remains one of the most studied tumor types in CAM systems due to its strong angiogenic profile and rapid growth [22]. Here, we outline the entire workflow from cell preparation and embryo windowing to tumor implantation, monitoring, histological processing, and downstream analysis. Using MDA-MB-231 and MCF-7 cells, this protocol enables the formation of measurable tumors within 72 h, allowing researchers to quickly assess tumor morphology and angiogenic characteristics. The model is also compatible with drug testing, gene silencing, overexpression, extracellular vesicle delivery, and co-culture with stromal or immune components [23,24].
Despite its advantages, the CAM system has limitations. Because embryonic development progresses rapidly, experiments must be completed within a limited timeframe, which prevents long-term studies of metastasis or tumor dormancy. Additionally, the immature immune system of the embryo hinders the evaluation of intricate adaptive immune responses. The relatively small size of the embryo and tumors may also restrict surgical manipulation and imaging resolution [25–27]. However, ongoing technical innovations (including intravital microscopy, micro-CT imaging, transcriptomic profiling, and nanoparticle tracking) are expanding the versatility of CAM-based approaches [23,28].
Overall, the CAM model provides an ethical, economical, and biologically relevant alternative for preclinical breast cancer research. Studies have demonstrated that CAM-derived tumors preserve histological architecture, proliferative markers, and angiogenic profiles closely resembling those observed in murine models and human patient specimens [23,29]. Consequently, this platform facilitates rapid, high-throughput in vivo experimentation while significantly reducing the use of mammalian animals [30]. Although the CAM model offers several experimental advantages, it also has intrinsic biological limitations that need to be considered. The experimental timeframe is limited, typically concluding around day 14 of embryonic development, which restricts long-term investigations of tumor growth, metastasis, and delayed therapeutic responses [31]. Furthermore, the chick embryo’s immunologically immature environment does not fully replicate the complex tumor–immune interactions observed in adult organisms, limiting its use for immunotherapy studies [32]. Therefore, the CAM assay should be viewed as a complementary preclinical platform rather than a substitute for established murine models. This protocol provides a practical, highly adaptable tool for studying tumor biology, evaluating therapeutic interventions, and connecting in vitro systems with mammalian models. While the primary focus of this study is breast cancer, the CAM model has also been successfully used to generate tumors from other cancer cell lines in our laboratory. These include neuroblastoma [SH-SY5Y, Be(2)-C], cervical cancer (HeLa, SiHa), lung cancer (H1299), and melanoma (A-375). This demonstrates the versatility of the CAM system and its potential applicability to a variety of cancer types.
Materials and reagents
Biological materials
1. Cell line MDA-MB-231 (ATCC® HTB-26)
2. Cell line MCF-7 (ATCC® HTB-22)
3. Fertilized hen eggs (Gallus gallus, Alpes, Puebla, México)
Reagents
1. Fetal bovine serum (FBS) (BIOWEST, catalog number: BIO-S1400-500); store at -20 °C; once thawed, keep at 4 °C and use within 1 month
2. DMEM/F12 (GIBCO, catalog number: 12500062 1L); store at 4 °C and use within 1 month after opening
3. Antibiotic-antimycotic 100× (AA) (BIOWEST, catalog number: BIO-L0010-100); store at -20 °C; once thawed, keep at 4 °C and use within 1 month
4. Trypsin-EDTA (0.25%) (GIBCO, catalog number: 25200072); store at 4 °C and use within 1 month after opening
5. Phosphate-buffered saline (PBS) (Fisher Scientific, catalog number: SH30256LS); store at 4 °C; once prepared, use within 1 month
6. Matrigel (CORNING, catalog number: 354234); thaw on ice and keep on ice during use; once thawed, keep at 4 °C and use within 1 month
7. Absolute ethanol (J.T. Baker, CAS No: 64-17-5); store at room temperature; stable for several years if tightly capped
8. Sodium bicarbonate (Sigma, catalog number: S5761-500G)
9. Trypan blue (HyClone, catalog number: SV30084.01)
Solutions
1. Supplemented culture medium (see Recipes)
2. 70% ethanol (see Recipes)
Recipes
1. Supplemented culture medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM/F12 | 89% | 89 mL |
| FBS | 10% | 10 mL |
| Antibiotic-antimycotic | 1% | 1 mL |
| Total | n/a | 100 mL |
Prepare 1,000 mL of basal DMEM/F12 culture medium according to the manufacturer’s instructions by dissolving each powder packet in 1 L of distilled water and adding 2.438 g/L of sodium bicarbonate. Sterilize the medium by filtration. The basal medium can be stored at 2–8 °C for up to four weeks. Prepare a 100 mL aliquot of supplemented medium by adding FBS and antibiotic-antimycotic and store the supplemented medium at 2–8 °C for up to four weeks.
2. 70% ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol absolute | 70% | 700 mL |
| Distilled water | 30% | 300 mL |
| Total | n/a | 1,000 mL |
Store at room temperature. Stable for several months in a tightly closed container.
Laboratory supplies
1. 100 × 20 mm cell culture plates (Santa Cruz Biotechnology, catalog number: SC251460)
2. 1.5 mL microtubes (Eppendorf, catalog number: 0030125150)
3. 10 mL serological pipettes (CORNING, catalog number: 511036)
4. 25 mL serological pipettes (CORNING, catalog number: 18293)
5. 1,000 μL pipette tips (BIOLOGIX, catalog number/SKU: 20-0200)
6. 200 μL pipette tips (BIOLOGIX, catalog number/SKU: 20-1000)
7. 15 mL conical tubes (Falcon, catalog number: 38009)
8. Style 2 precision jeweler’s tweezers, firm, non-magnetic stainless steel (MILTEX, catalog number/SKU: EF7228B)
9. Straight dissection forceps, 13 cm, stainless steel, without teeth (Hergom Medical, catalog number/SKU: 4-268-4)
10. Fine dissection scissors, straight, sharp–sharp, stainless steel, 22 mm cutting edge, 90 mm length (Fine Science Tool, catalog number/SKU: FINES01410)
11. Bottle top vacuum filter, 0.22 μm (Corning, catalog number: 430513)
Equipment
1. Automatic egg-turning incubator (closed eggs) (CASSER, model: 200 CASSER)
2. Non-turning egg incubator (windowed eggs) (LABLINE, model: 460)
3. Laboratory centrifuge (BECKMAN, model; Spinchron Benchtop Centrifuge)
4. Water bath (Chicago Surgical & Electrical Co., model: 26100)
5. Cell culture incubator (37 °C, 5% CO2) (NUAIRE, model: NU-450)
6. Inverted microscope for cell culture (Olympus, model: CK2)
7. Neubauer chamber (Isolab, model: 075.03.001)
8. Class II biological safety cabinet (Forma Scientific, model: 1284)
9. Stereomicroscope (Carl Zeiss, model: Stemi 2000-C)
Procedure
文章信息
稿件历史记录
提交日期: Nov 10, 2025
接收日期: Jan 4, 2026
在线发布日期: Jan 15, 2026
出版日期: Feb 20, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Patiño Morales, C. C., González de la Rosa, C. H., Jaime-Cruz, R., Salazar-García, M., Villavicencio Guzmán, L. and Herrera-Vargas, A. K. (2026). In Ovo CAM-Based Xenograft Model for Investigating Tumor Developmental Biology in Breast Cancer. Bio-protocol 16(4): e5600. DOI: 10.21769/BioProtoc.5600.
分类
癌症生物学 > 通用技术 > 动物模型
细胞生物学 > 细胞移植 > 异种移植
癌症生物学 > 血管生成
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












