- EN - English
- CN - 中文
On-Column Dual-Gradient Refolding for Efficient Recovery of Insoluble Affinity-Tagged Recombinant Proteins
柱上双梯度复性策略实现不溶性亲和标签重组蛋白的高效回收
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5598 浏览次数: 37
评审: Anonymous reviewer(s)
Abstract
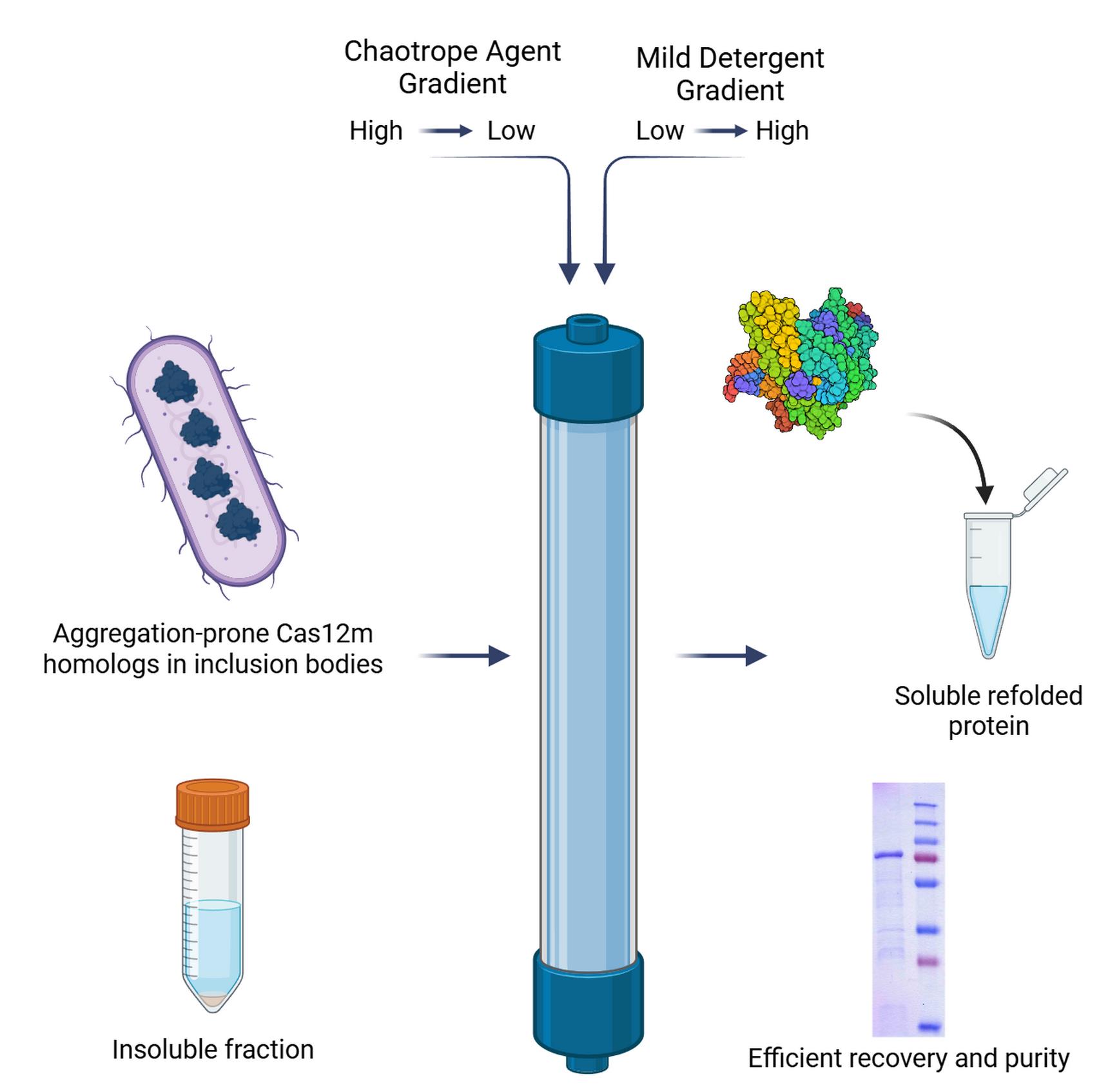
This article presents an efficient protocol for refolding recombinant proteins that are prone to aggregation and form inclusion bodies during expression in Escherichia coli. As a model system, the homolog of CRISPR-associated effector protein CasV-M was investigated. The key element of the developed approach is refolding directly on a metal-affinity Ni-TED (N,N,N´-tris(carboxymethyl)ethylendiamine) resin using a dual-gradient system: a stepwise reduction in the concentration of the chaotropic agent combined with a simultaneous increase in the concentration of a mild nonionic detergent. This combination ensures spatial separation of protein molecules, minimizes aggregation, and promotes the recovery of the native conformation. The resulting method appears to be an alternative to conventional refolding strategies, with potential improvements in the reproducibility and yield of soluble protein compared to dialysis or dilution. The proposed approach can be extended to a broad range of aggregation-prone proteins and is considered a promising strategy for obtaining otherwise insoluble recombinant proteins.
Key features
• This protocol requires optimizing E. coli protein expression and an FPLC system, being particularly suitable for insoluble proteins that form inclusion bodies.
• The method utilizes a dual-gradient refolding strategy on a Ni-TED column, integrating solubilization, refolding, and initial purification into a single workflow.
• Effectively rescues challenging proteins from inclusion bodies, demonstrated with aggregation-prone CRISPR-associated effector homologs (Cas12m) refractory to traditional refolding methods.
• Utilizes Ni-TED resin for its compatibility with high β-mercaptoethanol concentrations and moderate binding affinity, reducing nonspecific binding of host cell proteins.
Keywords: Recombinant protein (重组蛋白)Graphical overview
Background
The production of recombinant proteins in heterologous expression systems is a cornerstone of modern molecular biology, biochemistry, and biotechnology. Among the available platforms, Escherichia coli remains one of the most widely used due to its simplicity, cost-effectiveness, and high yield of the target product [1]. However, a major limitation of this system is the frequent formation of inclusion bodies (IBs), insoluble aggregates of misfolded protein [2]. This is particularly common for complex proteins, such as those containing disulfide bonds, multiple subunits, or prosthetic groups, as the intracellular environment of E. coli often cannot support their correct folding [3,4]. While IBs can offer protection from proteolysis and facilitate initial purification, the target protein must be solubilized, refolded, and purified to regain biological activity—a process that remains a significant bottleneck [5].
Traditional methods for refolding proteins from IBs include dilution and dialysis. These techniques rely on reducing the concentration of chaotropic agents used for solubilization (e.g., urea or guanidine hydrochloride) to allow the protein to regain its native conformation [6,7]. However, these methods often suffer from low efficiency and poor reproducibility, primarily due to protein aggregation at intermediate stages of denaturant removal, which is highly dependent on protein concentration and refolding conditions [8].
Chromatographic refolding strategies have emerged as powerful alternatives to overcome these limitations. By immobilizing the denatured protein on a chromatography resin, molecules are spatially separated, thereby minimizing intermolecular aggregation and favoring correct folding [9]. Ion exchange [10,11] and immobilized metal-affinity chromatography (IMAC) [12–14] have been successfully used for this purpose. A particularly effective approach involves the simultaneous application of two gradients during the refolding process: a decreasing gradient of the chaotropic agent and an increasing gradient of a mild detergent. This dual-gradient method helps to suppress aggregation by shielding hydrophobic patches exposed on the protein surface during the critical refolding phase [5,15].
This protocol describes an efficient on-column refolding method using IMAC with a dual-gradient system, developed for a homolog of the Cas12m protein representing subtype CRISPR-CasVM effectors (Dataset S1) [16–18]. Cas12m proteins form binary complexes with crRNA that are capable of binding to DNA with a 5'-TTN-3' PAM but are unable to hydrolyze DNA. The commonly utilized protocol for Cas12m isolation (often with modifications) describes a purification procedure for non-aggregated, soluble proteins [19]. The homolog studied in this work consistently formed inclusion bodies in E. coli across various expression strains and conditions, and traditional refolding methods proved ineffective. The present protocol, utilizing Ni-TED resin and a sodium lauroyl sarcosinate/Tween-20 gradient, enabled the successful production of soluble, albeit partially pure, protein suitable for downstream functional studies. The choice of Ni-TED is due to its high resistance to reducing agents, chelators, and alkaline conditions, which is critical for working with denaturing buffers. Furthermore, the capacity of this resin is lower than that of Ni-NTA (one Ni ion binds only one protein molecule), providing more specific binding compared to polydentate Ni-NTA, reducing nonspecific interactions and increasing the purity of the eluate. The detergent combination was optimized empirically: lauroyl sarcosinate effectively solubilizes inclusion bodies, while the nonionic Tween-20, being transparent at 280 nm, does not interfere with spectrophotometric protein concentration determination. The key advantages of this method over traditional dilution or dialysis are its enhanced reproducibility, reduced aggregation, and the integration of refolding with an initial purification step. This strategy can be adapted for a wide range of other aggregation-prone recombinant proteins that are resistant to conventional refolding approaches.
Materials and reagents
Biological materials
1. E. coli Rosetta 2 (DE3) pLysS (Novagen, catalog number: 71403)
Reagents
1. LB-Miller broth (Difco, catalog number: 244620)
2. Chloramphenicol (Sigma-Aldrich, catalog number: C0378)
3. Kanamycin sulfate (Sigma-Aldrich, catalog number: 60615)
4. Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, catalog number: I6758)
5. Tris-base (Fisher BioReagents, catalog number: BP152-500)
6. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
7. TritonTM X-100 (Sigma-Aldrich, catalog number: X100)
8. Benzonase® nuclease (Sigma-Aldrich, catalog number: E1014)
9. Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266)
10. β-Mercaptoethanol (β-ME) (Sigma-Aldrich, catalog number: M6250)
11. Imidazole (Sigma-Aldrich, catalog number: I2399)
12. Sodium lauroyl sarcosinate (Sigma-Aldrich, catalog number: 61747)
13. Tween® 20 (Sigma-Aldrich, catalog number: P9416)
14. Glycerol (Sigma-Aldrich, catalog number: G5516)
15. Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884)
16. Ethanol, 96% (Sigma-Aldrich, catalog number: 1.00971)
17. Acetic acid, glacial (Sigma-Aldrich, catalog number: A6283)
18. Coomassie Brilliant Blue R-250 (Thermo Scientific, catalog number: 20278)
19. TEMED (Sigma-Aldrich, catalog number: T9281)
20. Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L3771)
21. Ammonium persulfate (APS) (Sigma-Aldrich, catalog number: A3678)
22. Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: 78830)
23. Prestained protein ladder PageRulerTM Plus, 10–250 kDa (Thermo Scientific, catalog number: 26619)
24. DL-Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: 43815)
25. Ni Seplife FF (TED) chromatography resin (Sunresin New Materials Co Ltd., catalog number: 20211218101)
26. Di-sodium hydrogen phosphate dihydrate (Na2HPO4·2H2O) (Himedia, catalog number: GRM1257)
27. Sodium acetate trihydrate (CH3COONa·3H2O) (Sigma-Aldrich, catalog number: 236500)
28. Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 795429)
29. Acrylamide (Sigma-Aldrich, catalog number: A8887)
30. N,N′-Methylenebisacrylamide (Sigma-Aldrich, catalog number: M7279)
Solutions
1. LB-Miller medium (see Recipes)
2. 1 M MgCl2 (see Recipes)
3. 1 M Tris-base, pH 8.0 (see Recipes)
4. 5 M NaCl (see Recipes)
5. Buffer 1 (see Recipes)
6. Buffer 2 (see Recipes)
7. Buffer 3 (see Recipes)
8. Buffer 4 (see Recipes)
9. Buffer 5 (see Recipes)
10. Buffer 6 (see Recipes)
11. Fixing solution (see Recipes)
12. 12% SDS-PAGE (see Recipes)
13. 50% (v/v) glycerol (see Recipes)
14. Kanamycin (50 mg/mL) (see Recipes)
15. Chloramphenicol (34 mg/mL) (see Recipes)
16. 0.5 M IPTG (see Recipes)
17. Coomassie brilliant blue stain (see Recipes)
18. 0.1 M EDTA, pH 8.0 (see Recipes)
19. 1 M imidazole pH 8.0 (see Recipes)
20. 30% Acrylamide/Bis solution (see Recipes)
21. 1.5 M Tris-Base, pH 8.8 (see Recipes)
22. 0.5 M Tris-Base, pH 6.8 (see Recipes)
23. 10% SDS (see Recipes)
24. 10% APS (see Recipes)
25. 1 M DTT (see Recipes)
26. 10% Triton X-100 solution (see Recipes)
27. 1 M NaOH (see Recipes)
28. Buffer 7 (see Recipes)
29. Buffer 8 (see Recipes)
Recipes
1. LB-Miller medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| LB-Miller broth powder | 25 g/L | 25 g |
| Milli-Q water | n/a | to 1 L |
Dissolve 25 g of LB-Miller broth powder in approximately 800 mL of Milli-Q water. Mix thoroughly until all components are completely dissolved. Adjust the final volume to 1 L with Milli-Q water. Sterilize by autoclaving at 121 °C for 20 min. Store the sterilized medium at room temperature.
2. 1 M MgCl2
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MgCl2·6H2O | 1 M | 101.65 g |
| Milli-Q water | n/a | to 500 mL |
Dissolve 101.65 g of MgCl2·6H2O in 400 mL of Milli-Q water. Once fully dissolved, bring the final volume to 500 mL with Milli-Q water. Filter the buffer using a 0.22 μm filter.
3. 1 M Tris-base, pH 8.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-base | 1 M | 121.14 g |
| Milli-Q water | n/a | to 1 L |
Dissolve 121.14 g of Tris-base in approximately 800–900 mL of Milli-Q water with vigorous stirring. Once completely dissolved, adjust the final volume to 1 L with Milli-Q water. Filter the solution through a 0.22 μm filter. The pH of the resulting solution is approximately 10.5 and must be adjusted to pH 8.0 using concentrated HCl before use for most applications.
4. 5 M NaCl
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | 5 M | 292.2 g |
| Milli-Q water | n/a | to 1 L |
Dissolve 292.2 g of NaCl in approximately 800 mL of Milli-Q water with stirring. Once completely dissolved, bring the final volume to 1 L with Milli-Q water. Filter the solution through a 0.22 μm filter. Store at room temperature.
5. Buffer 1
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-base, pH 8.0 | 40 mM | 40 mL |
| 5 M NaCl | 400 mM | 80 mL |
| 10% Triton X-100 | 0.2% | 2 mL |
| Milli-Q water | n/a | to 1 L |
Add approximately 800 mL of Milli-Q water to a 1 L graduated cylinder or beaker. Add 80 mL of 5 M NaCl stock solution, 40 mL of 1 M Tris-base stock solution (pH 8.0), and 2 mL of 10% Triton X-100 solution. Mix thoroughly until all components are completely dissolved and the solution appears homogeneous. Adjust the final volume to 1 L with Milli-Q water, verify that the pH is 7.8, and adjust if necessary using diluted HCl or NaOH. Filter the buffer through a 0.22 μm filter and store at 4 °C for up to 1 month.
6. Buffer 2
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-base, pH 8.0 | 40 mM | 40 mL |
| 5 M NaCl | 400 mM | 80 mL |
| Milli-Q water | n/a | to 1 L |
Add approximately 800 mL of Milli-Q water to a 1 L graduated cylinder or beaker. Add 80 mL of 5 M NaCl stock solution and 40 mL of 1 M Tris-base stock solution (pH 8.0). Mix thoroughly until the solution is homogeneous. Adjust the final volume to 1 L with Milli-Q water, verify that the pH is 7.8, and adjust if necessary using diluted HCl or NaOH. Filter the buffer through a 0.22 μm filter and store at 4 °C for up to 6 months.
7. Buffer 3
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-base, pH 8.0 | 40 mM | 40 mL |
| 5 M NaCl | 400 mM | 80 mL |
| 1 M imidazole | 10 mM | 10 mL |
| Sodium lauroyl sarcosinate | 1% (w/v) | 10 g |
| β-ME | 10 mM | 0.714 mL |
| Milli-Q water | n/a | to 1 L |
Add approximately 700 mL of Milli-Q water to a 1 L graduated cylinder or beaker. Add 80 mL of 5 M NaCl stock solution, 40 mL of 1 M Tris-base stock solution (pH 8.0), 10 mL of 1 M imidazole stock solution, and 10 g of sodium lauroyl sarcosinate powder. Mix thoroughly until all components, particularly the detergent powder, are completely dissolved. Add 1 mL of β-ME and mix well. Adjust the final volume to 1 L with Milli-Q water, verify that the pH is 7.8, and adjust if necessary using diluted HCl or NaOH. Filter the buffer through a 0.22 μm filter and store at 4 °C for up to 1 month.
Note: Due to the volatility and oxidation of β-ME, it is recommended to add this component fresh immediately before use if the buffer is stored for more than a few days. The chaotropic agent in this buffer can be optimized (urea 4–8 M or guanidine hydrochloride 4–6 M).
8. Buffer 4
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-base, pH 8.0 | 40 mM | 40 mL |
| 5 M NaCl | 400 mM | 80 mL |
| 1 M imidazole, pH 8.0 | 10 mM | 10 mL |
| Tween-20 | 0.1% (v/v) | 1 mL |
| β-ME | 10 mM | 0.714 mL |
| Glycerol (100%) | 5% (v/v) | 50 mL |
| Milli-Q water | n/a | to 1 L |
Add approximately 700 mL of Milli-Q water to a 1 L graduated cylinder or beaker. Add 80 mL of 5 M NaCl stock solution, 40 mL of 1 M Tris-base stock solution (pH 8.0), and 10 mL of 1 M imidazole stock solution. Mix thoroughly until all components are completely dissolved. Add 1 mL of β-ME and 1 mL of Tween-20, then mix well to ensure complete homogeneity. Adjust the final volume to 1 L with Milli-Q water, verify that the pH is 7.8, and adjust if necessary using diluted HCl or NaOH. Filter the buffer through a 0.22 μm filter and store at 4 °C for up to 1 month.
Note: Due to the volatility and oxidation of β-ME, it is recommended to add this component fresh immediately before use if the buffer is stored for more than a few days.
9. Buffer 5
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-base, pH 8.0 | 40 mM | 40 mL |
| 5 M NaCl | 400 mM | 80 mL |
| 1 M imidazole, pH 8.0 | 300 mM | 300 mL |
| Glycerol (100%) | 5% (v/v) | 50 mL |
| Tween-20 | 0.1% (v/v) | 1 mL |
| β-ME | 5 mM | 0.357 mL |
| Milli-Q water | n/a | to 1 L |
Add approximately 400 mL of Milli-Q water to a 1 L graduated cylinder or beaker. Add 80 mL of 5 M NaCl stock solution, 40 mL of 1 M Tris-base stock solution (pH 8.0), 300 mL of 1 M imidazole stock solution, and 50 mL of glycerol. Mix thoroughly until the solution is homogeneous. Add 1 mL of Tween-20 and 5 mL of β-ME, then mix well to ensure complete homogeneity. Adjust the final volume to 1 L with Milli-Q water, verify that the pH is 7.8, and adjust if necessary using diluted HCl or NaOH. Filter the buffer through a 0.22 μm filter and store at 4 °C for up to 1 month.
Note: Due to the volatility and oxidation of β-ME, it is recommended to add this component fresh immediately before use if the buffer is stored for more than a few days.
10. Buffer 6
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-base, pH 8.0 | 40 mM | 40 mL |
| 5 M NaCl | 400 mM | 80 mL |
| 0.1 M EDTA | 5 mM | 50 mL |
| Glycerol (100%) | 5% (v/v) | 50 mL |
| Tween-20 | 0.1% (v/v) | 1 mL |
| β-ME | 5 mM | 0.357 mL |
| Milli-Q water | n/a | to 1 L |
Add approximately 500 mL of Milli-Q water to a 1 L graduated cylinder or beaker. Add 80 mL of 5 M NaCl stock solution, 40 mL of 1 M Tris-base stock solution (pH 8.0), 50 mL of 0.1 M EDTA stock solution, and 50 mL of glycerol. Mix thoroughly until the solution is homogeneous. Add 1 mL of Tween-20 and 0.35 mL of β-ME, then mix well to ensure complete homogeneity. Adjust the final volume to 1 L with Milli-Q water, verify that the pH is 7.8, and adjust if necessary using diluted HCl or NaOH. Filter the buffer through a 0.22 μm filter and store at 4 °C for up to 1 month.
Note: Due to the volatility and oxidation of β-ME, it is recommended to add this component fresh immediately before use if the buffer is stored for more than a few days.
11. Fixing solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol (96%) | 40% (v/v) | 416.7 mL |
| Glacial acetic acid | 10% (v/v) | 100 mL |
| Milli-Q water | n/a | to 1 L |
Add approximately 300 mL of Milli-Q water to a 1 L graduated cylinder placed in a well-ventilated area or fume hood. Carefully add 416.7 mL of 96% ethanol and 100 mL of glacial acetic acid. Mix thoroughly until the solution is homogeneous. Adjust the final volume to 1 L with Milli-Q water. Store at room temperature in a tightly sealed container to prevent evaporation.
Caution: Prepare this solution in a well-ventilated area or fume hood due to the volatile and corrosive nature of glacial acetic acid. Wear appropriate personal protective equipment.
12. 12% SDS-PAGE (for 2 mini-gels)
a. Resolving gel (10 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 30% acrylamide/bis solution | 12% | 4.0 mL |
| 1.5 M Tris-base, pH 8.8 | 0.57 M | 3.8 mL |
| 10% SDS | 0.15% | 150 μL |
| 10% APS | 0.15% | 150 μL |
| TEMED | 0.06% | 6 μL |
| Milli-Q water | n/a | 2.9 mL |
Combine 4.0 mL of 30% acrylamide/bis solution, 3.8 mL of 1.5 M Tris-base (pH 8.8), 150 μL of 10% SDS, 2.9 mL of Milli-Q water, and 150 μL of 10% APS in a 15 mL conical tube. Mix gently by inversion. Immediately before pouring, add 6 μL of TEMED, mix gently but thoroughly, and pour the solution immediately into the gel cassette. Leave appropriate space for the stacking gel and carefully overlay with isopropanol or water to ensure a flat interface. Allow to polymerize completely (approximately 15–30 min).
Caution: Acrylamide is a neurotoxin. Wear appropriate personal protective equipment when handling unpolymerized solutions.
b. Stacking gel (4 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 30% acrylamide/bis solution | 5% | 670 μL |
| 0.5 M Tris-base, pH 6.8 | 0.125 M | 1.0 mL |
| 10% SDS | 0.1% | 40 μL |
| 10% APS | 0.1% | 40 μL |
| TEMED | 0.1% | 4 μL |
| Milli-Q water | n/a | 2.25 mL |
After polymerization of the resolving gel, remove the overlay and rinse the gel surface with Milli-Q water. Combine 670 μL of 30% acrylamide/bis solution, 1.0 mL of 0.5 M Tris-base (pH 6.8), 40 μL of 10% SDS, 2.25 mL of Milli-Q water, and 40 μL of 10% APS in a 15 mL conical tube. Mix gently by inversion. Add 4 μL of TEMED, mix gently but thoroughly, and pour immediately on top of the polymerized resolving gel. Immediately insert a clean comb, avoiding air bubbles. Allow to polymerize completely (approximately 10–15 min).
Caution: Acrylamide is a neurotoxin. Wear appropriate personal protective equipment when handling unpolymerized solutions.
13. 50% (v/v) glycerol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Molecular biology–grade glycerol | 50% (v/v) | 50 mL |
| Milli-Q water | n/a | 50 mL |
Add 50 mL of molecular biology–grade glycerol to 50 mL of Milli-Q water in a suitable container. Mix thoroughly on a magnetic stirrer until the solution is completely homogeneous. Store at room temperature.
14. Kanamycin (50 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Kanamycin sulfate | 50 mg/mL | 2.5g |
| Sterile Milli-Q water | n/a | to 50 mL |
Dissolve 2.5 g of kanamycin sulfate in approximately 40 mL of sterile Milli-Q water. After complete dissolution, adjust the final volume to 50 mL with sterile Milli-Q water. Sterilize the solution by filtration through a 0.22 μm filter. Aliquot into sterile tubes and store at -20 °C.
15. Chloramphenicol (34 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Chloramphenicol | 34 mg/mL | 1.7 g |
| Absolute ethanol | 100% (v/v) | 50 mL |
Dissolve 1.7 g of chloramphenicol in 50 mL of absolute ethanol. Mix thoroughly until completely dissolved. The solution is sterile and does not require filtration. Aliquot and store at -20 °C protected from light.
16. 0.5 M IPTG
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| IPTG | 0.5 M | 5.95 g |
| Milli-Q water | n/a | to 50 mL |
Dissolve 5.95 g of IPTG in approximately 40 mL of Milli-Q water. After complete dissolution, adjust the final volume to 50 mL with Milli-Q water. Sterilize the solution by filtration through a 0.22 μm filter. Aliquot into sterile tubes and store at -20 °C.
17. Coomassie Brilliant Blue staining solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Coomassie Brilliant Blue R-250 | 0.1% (w/v) | 1.0 g |
| Ethanol (absolute) | 45% (v/v) | 450 mL |
| Glacial acetic acid | 10% (v/v) | 100 mL |
| Milli-Q water | n/a | 450 mL |
Dissolve 1.0 g of Coomassie Brilliant Blue R-250 in a mixture of 450 mL of absolute ethanol and 100 mL of glacial acetic acid, using a magnetic stirrer until the dye is completely dissolved. Add 450 mL of Milli-Q water and mix thoroughly until homogeneous. Filter the solution through filter paper before use to remove any undissolved particles. Store at room temperature in a tightly sealed container.
Caution: Glacial acetic acid is corrosive and volatile. Prepare this solution in a well-ventilated area or fume hood and wear appropriate personal protective equipment.
18. 0.1 M EDTA, pH 8.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| EDTA disodium salt dihydrate | 0.1 M | 37.22 g |
| Milli-Q water | n/a | to 1 L |
Dissolve 37.22 g of EDTA disodium salt dihydrate in approximately 800 mL of Milli-Q water while stirring vigorously. Adjust the pH to 8.0 using NaOH pellets or a concentrated (e.g., 1 M) NaOH solution; note that the EDTA will not fully dissolve until the pH approaches 8.0. Once completely dissolved and at the correct pH, adjust the final volume to 1 L with Milli-Q water. The solution can be sterilized by autoclaving at 121 °C for 20 min if required. Store at room temperature.
19. 1 M imidazole, pH 8.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Imidazole | 1 M | 34.04 g |
| Milli-Q water | n/a | to 500 mL |
Dissolve 34.04 g of imidazole in approximately 400 mL of Milli-Q water. While stirring, adjust the pH to 8.0 using concentrated HCl. Once the pH is stable and the imidazole is completely dissolved, adjust the final volume to 500 mL with Milli-Q water. Filter-sterilize the solution through a 0.22 μm filter and store at room temperature.
20. 30% acrylamide/bis solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acrylamide | 29.2% (w/v) | 146 g |
| N, N'-methylenebisacrylamide | 0.8% (w/v) | 4 g |
| Milli-Q water | n/a | to 500 mL |
Dissolve 146 g of acrylamide and 4 g of N, N'-methylenebisacrylamide in approximately 400 mL of Milli-Q water with gentle stirring. Once completely dissolved, adjust the final volume to 500 mL with Milli-Q water. Filter the solution through a 0.45 μm filter. Store in a dark bottle or container wrapped in aluminum foil at 4 °C to protect from light.
Caution: Acrylamide is a potent neurotoxin and a suspected carcinogen. Always wear appropriate personal protective equipment (gloves, lab coat, safety glasses) when handling unpolymerized acrylamide. Avoid inhalation of powder and skin contact.
21. 1.5 M Tris-base, pH 8.8
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-base | 1.5 M | 90.85 g |
| Milli-Q water | n/a | to 500 mL |
Dissolve 90.85 g of Tris-base in approximately 400 mL of Milli-Q water. While stirring, adjust the pH to 8.8 using concentrated HCl. Note that a significant volume of HCl may be required, and the solution will generate heat during neutralization. Allow the solution to cool to room temperature after pH adjustment, then check and adjust the pH again if necessary. Once the pH is stable at 8.8 and the Tris-base is completely dissolved, adjust the final volume to 500 mL with Milli-Q water. The solution can be sterilized by autoclaving at 121 °C for 20 min if required. Store at room temperature.
22. 0.5 M Tris-base, pH 6.8
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-base | 0.5 M | 30.3 g |
| Milli-Q water | n/a | to 500 mL |
Dissolve 30.3 g of Tris-base in approximately 400 mL of Milli-Q water. While stirring, carefully adjust the pH to 6.8 using concentrated HCl. The solution will generate heat during neutralization; allow it to cool to room temperature before making final pH adjustments. Once the pH is stable at 6.8 and the Tris-base is completely dissolved, adjust the final volume to 500 mL with Milli-Q water. The solution can be sterilized by autoclaving at 121 °C for 20 min if required. Store at room temperature.
23. 10% SDS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| SDS | 10% (w/v) | 50 g |
| Milli-Q water | n/a | to 500 mL |
Dissolve 50 g of SDS in approximately 400 mL of Milli-Q water with gentle stirring and heating to 50 °C to facilitate dissolution. Once fully dissolved and when the solution appears clear, adjust the final volume to 500 mL with Milli-Q water. Store at room temperature.
Caution: Wear appropriate personal protective equipment, including a mask, when handling SDS powder to avoid inhalation.
24. 10% APS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| APS | 10% (w/v) | 1 g |
| Milli-Q water | n/a | 10 mL |
Dissolve 1 g of APS in 10 mL of Milli-Q water. Mix thoroughly until completely dissolved. Aliquot into small volumes (e.g., 0.5–1 mL) and store at -20 °C.
Note: The solution is stable for several weeks when stored frozen. For best results in polymerization reactions, use freshly prepared solution or aliquots that have undergone minimal freeze-thaw cycles.
25. 1 M DTT
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DTT | 1 M | 1.54 g |
| Milli-Q water | n/a | to 10 mL |
Dissolve 1.54 g of DTT in approximately 8 mL of Milli-Q water. Once fully dissolved, adjust the final volume to 10 mL with Milli-Q water. Sterilize the solution by filtration through a 0.22 μm filter. Aliquot into small working volumes and store at -20 °C.
Note: DTT solutions are susceptible to oxidation. For critical applications, prepare fresh solutions or use aliquots that have undergone minimal freeze-thaw cycles.
26. 10% Triton X-100 solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Triton X-100 | 10% (v/v) | 10 mL |
| Milli-Q water | n/a | to 100 mL |
Add 10 mL of Triton X-100 to approximately 80 mL of Milli-Q water. Mix thoroughly using a magnetic stirrer until the solution becomes clear and homogeneous; this may take several minutes. Adjust the final volume to 100 mL with Milli-Q water. Store at 4 °C.
27. 1 M NaOH
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaOH | 1 M | 4 g |
| Milli-Q water | n/a | to 100 mL |
Measure approximately 80 mL of water into a volumetric flask or beaker.
Caution: Add 4.0 g of NaOH while stirring. Dissolution is highly exothermic; the solution will become hot. Allow the solution to cool to room temperature. Bring the volume up to the 100 mL mark with water. Mix thoroughly.
28. Buffer 7
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Na2HPO4·2H2O | 0.4 M | 7.1 g |
| NaCl | 0.15 M | 0.88 g |
| Milli-Q water | n/a | to 100 mL |
Measure approximately 80 mL of water into a volumetric flask. Dissolve 7.1 g of Na2HPO4·2H2O in it. Add 0.88 g of NaCl and stir until completely dissolved. Check the pH of the solution. The Na2HPO4 solution will have an alkaline pH (usually ~8.9–9.0). Slowly add concentrated HCl dropwise while stirring and monitoring with a pH meter, until the pH reaches exactly 7.0.
Tip: It is better to dilute a small portion of HCl (e.g., to 1 M) for more precise adjustment.
Bring the volume up to the 100 mL mark with water.
29. Buffer 8
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CH3COONa·3H2O | 0.4 M | 0.14 g |
| Ethanol | 20% | 20 mL |
| Milli-Q water | n/a | to 100 mL |
Prepare 10 mM acetate buffer, pH 6.5: Dissolve 1.36 g of CH3COONa·3H2O in approximately 20 mL of water. Measure the pH. The sodium acetate solution will have an alkaline pH. Slowly, while stirring and monitoring with a pH meter, add acetic acid until the pH becomes 6.5. Bring the volume of this buffer up to 80 mL with water. Mix well. To 80 mL of the finished acetate buffer (pH 6.5), add 20 mL of ethanol. Mix thoroughly.
Laboratory supplies
1. Safe-lock tubes, 0.5 mL (Eppendorf, catalog number: 0030121023)
2. Safe-lock tubes, 1.5 mL (Eppendorf, catalog number: 0030120086)
3. Safe-lock tubes, 2.0 mL (Eppendorf, catalog number: 0030120094)
4. Falcon conical centrifuge tubes, 15 mL (Corning, catalog number: 352095)
5. Falcon conical centrifuge tubes, 50 mL (Corning, catalog number: 352070)
6. Dialysis tubing SnakeSkin, 10 K MWCO (Thermo Scientific, catalog number: 68100)
7. Centrifugal filters JetSpin, 10 KDa MWCO (JetBioFil, catalog number: FTT410500)
8. Serological pipettes, 10 mL (GenFollower, catalog number: YSPS10-1)
9. Petri dishes (Sigma-Aldrich, catalog number: P5481-500EA)
10. Pipette tips (various volumes) (Eppendorf, catalog numbers: EP0030000870-1PAK, EP0030078578-960EA, EP0030078519-960EA)
11. Volumetric flasks (Glassco, catalog numbers: Q138.510.01, Q138.510.04, Q138.510.06)
12. Beakers (MiniMed, catalog numbers: В-1-150, В-1-250, Н-1-600)
13. Erlenmeyer flasks (HARIO, catalog number: SF-1L SCI)
14. Calibrated pipettes (various volumes) (DLAB TopPette, catalog number: 7010101014, LH-7290207010101009)
Equipment
1. Water bath thermostat ELMI TW-2 (ELMI, catalog number: TW-2)
2. Standard incubator binder BD 56 (Binder, catalog number: 9010-0323)
3. Biological safety cabinet Labconco Purifier Logic+ Class II, Type A2 (Labconco, catalog number: 302481101)
4. Stackable incubator shaker Innova® S44i (Eppendorf, catalog number: S44I300001)
5. Spectrophotometer SmartSpec Plus (Bio-Rad, catalog number: 170-2525)
6. Portable mini centrifuge Eppendorf MiniSpin (Eppendorf, catalog number: 5452000010)
7. High-speed centrifuge Beckman Coulter Avanti J-E (Beckman Coulter, catalog number: 369001)
8. High-speed benchtop centrifuge Eppendorf Centrifuge 5804R (Eppendorf, catalog number: 5805000010)
9. Digital sonifier Branson 450 (Branson, catalog number: 100-132-889R)
10. Empty chromatography column 5 mL (Smart-Lifesciences, catalog number: SLM011)
11. Chromatography system ÄKTA Pure (Cytiva, catalog number: 29018226)
12. Magnetic stirrer Heidolph MR Hei-Standard (Heidolph, catalog number: 505-20000-00)
13. Vertical polyacrylamide gel electrophoresis system Bio-Rad Mini-PROTEAN Tetra Cell (Bio-Rad, catalog number: 1658005EDU)
14. Electrophoresis power supply Bio-Rad PowerPac Basic (Bio-Rad, catalog number: 1645050)
15. Thermo-shaker with cooling BioSan TS-100C (BioSan, catalog number: BS-010143-AAI)
16. Spectrophotometer Thermo Fisher Scientific NanoDrop 1000 (Thermo Fisher Scientific, catalog number: ND1000)
17. Ultra-low temperature freezer Sanyo MDF-U53V (Sanyo, catalog number: SM9910073)
18. Water purification system Rephile NuZar Q (Rephile, catalog number: RN0Q00000K)
19. pH-meter Mettler Toledo S20 SevenEasyTM (Mettler Toledo, catalog number: S20)
20. High-pressure steam sterilizer (autoclave) Sanyo MLS-3781L (Sanyo, catalog number: SM6610004)
21. Lab balance Mettler Toledo XPE204 (Mettler Toledo, catalog number: 30087643)
22. Sterile disposable filter unit Nalgene Rapid-Flow (Thermo Fisher Scientific, catalog number: 566-0020)
23. Freezer (-20 °C) ХЛ-250 POZIS (Pozis, catalog number: 223TV)
24. Refrigerator (2–8 °C) ХЛ-250 POZIS (Pozis, catalog number: 223TV)
Software and datasets
1. UNICORN 7 control software (Cytiva)
2. Image Lab Software (Bio-Rad)
Procedure
文章信息
稿件历史记录
提交日期: Oct 19, 2025
接收日期: Dec 25, 2025
在线发布日期: Jan 15, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Vlaskina, A., Petrenko, D., Agapova, Y., Kuzminkova, A., Evteeva, M. and Patrushev, M. (2026). On-Column Dual-Gradient Refolding for Efficient Recovery of Insoluble Affinity-Tagged Recombinant Proteins. Bio-protocol 16(3): e5598. DOI: 10.21769/BioProtoc.5598.
分类
生物化学 > 蛋白质 > 分离和纯化
微生物学 > 异源表达系统 > 大肠杆菌
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











