- EN - English
- CN - 中文
Isolation and Transfection of Protoplasts From Maize Mesophyll Cells
玉米叶肉细胞原生质体的分离与转染方法
(*contributed equally to this work) 发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5596 浏览次数: 100
评审: Anonymous reviewer(s)
Abstract
Protoplast systems are widely used in plant research as versatile platforms for studying cellular processes and validating gene editing tools. In maize, they are particularly valuable because stable transformation in immature embryos is slow and labor-intensive, often requiring months to regenerate plants. However, existing protocols often yield inconsistent results in protoplast recovery, transfection efficiency, and viability. We present an optimized protocol for maize mesophyll protoplast isolation and PEG-mediated transfection. Two-week-old etiolated seedlings are processed using vertical cutting, improving the yield and viability of protoplasts. Protoplasts are then immediately transformed with a CRISPR/Cas9 construct after isolation, via PEG4000 with only 10 μg of plasmid DNA, reducing the resource demands of standard methods. Modified washing and storage conditions extend transformed protoplast viability to seven days, enabling longer-term monitoring and expanded downstream analyses. Editing outcomes are quantified by sequencing target sites and calculating efficiency with Cas-Analyzer. This protocol provides a rapid, efficient, and reproducible method for the rapid evaluation of gene editing in maize. This protocol offers a methodology to accelerate agricultural crop studies and broader plant molecular biology.
Key features
• Optimized maize mesophyll protoplast isolation using vertical cutting to improve yield, consistency, and viability.
• Efficient PEG4000-mediated transformation requiring only 10 μg of plasmid DNA for CRISPR delivery.
• Extended protoplast viability up to seven days through modified washing and storage conditions, enabling longer monitoring and analysis.
• Rapid and reproducible gene-editing evaluation via targeted sequencing and Cas-Analyzer quantification.
Keywords: Protoplast (原生质体)Graphical overview
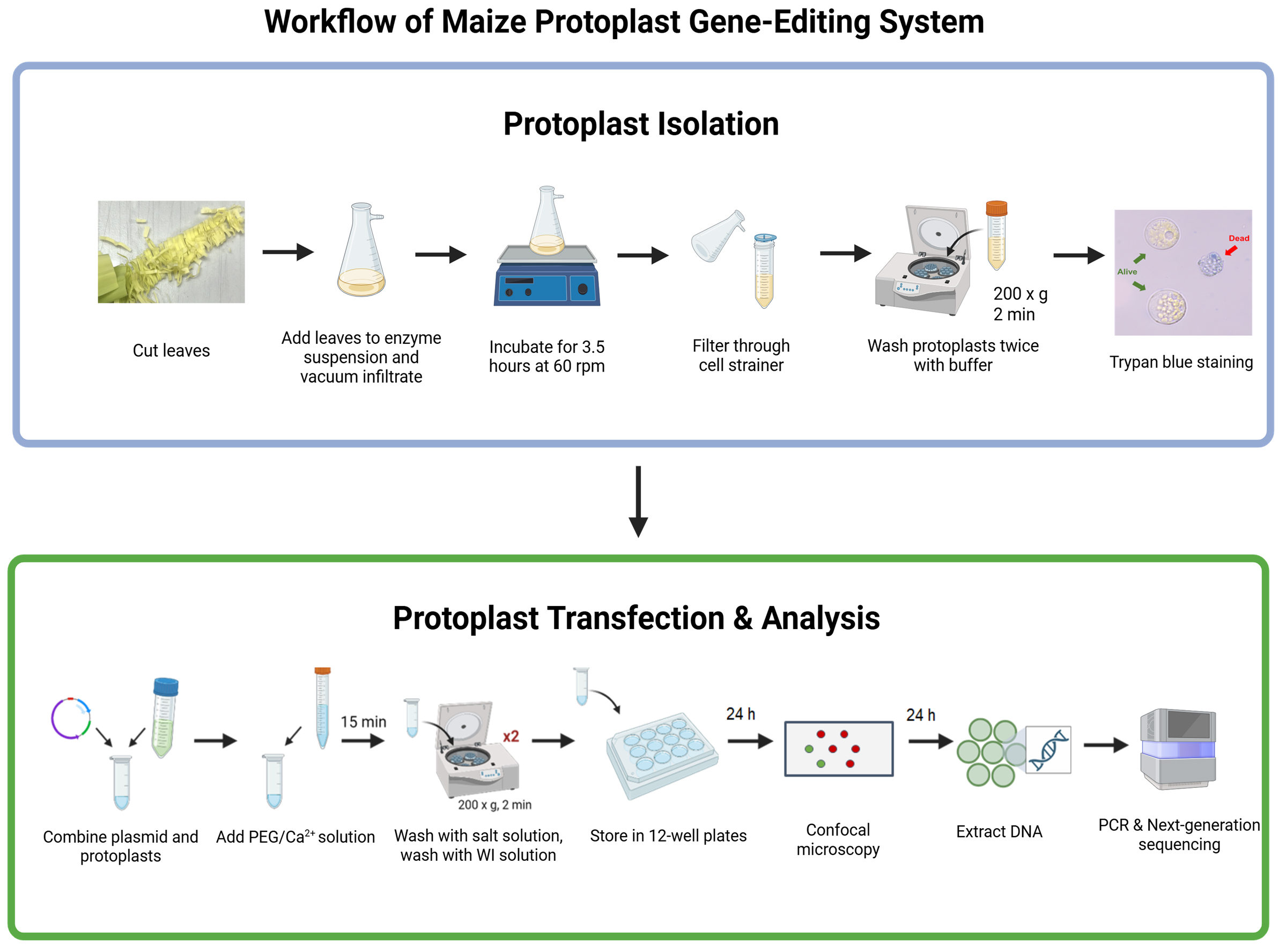
Background
Genetic engineering of crops is an important strategy for improving yield, nutritional quality, and resilience to environmental stress. CRISPR/Cas9 has become a central tool in this work, yet the efficiency of guide RNAs (gRNAs) can be strongly influenced by chromatin accessibility, GC content, and local nucleotide context [1–4]. Stable transformation in maize remains a slow and resource-intensive process, often taking eight to nine months to regenerate plants and evaluate gene editing events [5]. As such, it is advantageous to evaluate gRNA performance before committing to stable transformation in immature embryos. Protoplast systems provide a practical solution, allowing gRNA activity to be tested in vivo in less than 1 week. However, existing protocols for maize protoplast isolation and transfection often produce variable results in yield, efficiency, and cell viability. For example, seedling growth conditions remain a point of debate, as many studies suggest that etiolated seedlings yield more viable protoplasts [6]. Additionally, cutting methods also influence recovery. While most protocols use horizontal leaf sections, vertical cutting has been shown to release more protoplasts per gram of fresh weight. This improved yield is especially valuable when plant material is limited. Transfection parameters often require high amounts of plasmid, with commonly used protocols requiring 20 μg of plasmid DNA, adding to the burden of plasmid preparation. Many established methods were designed for short-term assays such as luminescence or protein localization, where protoplast viability beyond 6–18 h is not necessary [7–9]. In contrast, gene editing requires cells to remain viable for at least 48 h to capture CRISPR-induced edits. The following protocol incorporates modifications to the methodologies presented in Yoo et al. (2007) and Gomez-Cano et al. (2019) [14,18] to improve yield, reduce DNA input, and extend protoplast viability. Although this protocol is described in the context of gene editing, these improvements can be applied to other monocot systems and adapted for various downstream applications. Additionally, extending protoplast viability allows for longer-term studies of cellular processes, such as protein localization [8,10], protein interactions [7,11], cell signaling [12,13], gene regulation [14], metabolomics [9], and transcriptomics [15]. Some considerations for the utilization of this protocol are that protoplasts cannot currently be regenerated into whole plants, and that edits introduced through non-homologous end joining (NHEJ) are random, so that sequence variants observed in protoplasts may not precisely predict edits recovered after stable transformation [16]. Despite this, characterizing gRNA efficiency in protoplasts is a critical evaluation tool, as high editing efficiency significantly increases the likelihood of generating desirable mutations in subsequent transformations.
Materials and reagents
Biological materials
1. TZI8 maize seeds (Maize Genetics Corporation Stock Center, TZI8)
Reagents
1. Bovine serum albumin (BSA) (Millipore Sigma, CAS: 9048-46-8)
2. Calcium chloride (CaCl2) (Fisher Scientific, CAS: 10043-52-4)
3. Cellulase (Millipore Sigma, CAS: 9012-54-8)
4. Cetyltrimethylammonium bromide (CTAB) (Fisher Scientific, CAS: 57-09-0)
5. Ethylenediaminetetraacetic acid, disodium salt dihydrate (EDTA) (Fisher Scientific, catalog number: S311-100)
6. Isopropanol, 99.5% (Fisher Scientific, CAS: 67-63-0)
7. Potassium chloride (KCl) (Fisher Scientific, CAS: 7447-40-7)
8. D-Mannitol (Fisher Scientific, CAS: 69-65-8)
9. Macerozyme (Research Products International, CAS: 9032-75-1)
10. 2-morpholin-4-ylethanesulfonic acid (MES) (Fisher Scientific, CAS: 145224-94-8)
11. Magnesium chloride (MgCl2) (Fisher Scientific, CAS: 7786-30-3)
12. Polyethylene glycol 4,000 (PEG 4000) (Fisher Scientific, catalog number: AAA1615130)
13. RNase A solution (Fisher Scientific, catalog number: PR-A7973)
14. Sodium chloride (NaCl) (Fisher Scientific, CAS: 7647-14-5)
15. 2-Amino-2-(hydroxymethyl) propane-1,3-diol (Tris-HCl) (Fisher Scientific, CAS: 77-86-1)
16. Trypan Blue solution, 0.4% (Fisher Scientific, catalog number: 15250061)
17. UltraPureTM phenol:chloroform:isoamyl alcohol (25:24:1, v/v) (Fisher Scientific, catalog number: 15593031)
Solutions
1. Protoplast buffer (see Recipes)
2. MMG buffer (see Recipes)
3. W5 buffer (see Recipes)
4. WI buffer (see Recipes)
5. PEG solution (see Recipes)
6. CTAB buffer (see Recipes)
7. Enzyme suspension (see Recipes)
Recipes
1. Protoplast buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| D-Mannitol (0.6 M) | 585 mM | 48.75 mL |
| KCl (2.0 M) | 10 mM | 0.25 mL |
| MES (0.5 M, pH 5.7) | 10 mM | 1.0 mL |
Add the reagents to a 50 mL Falcon tube and mix gently by inversion at room temperature. Prepare on the day of the experiment; do not save leftover buffer. Buffer should be stored at room temperature; it expires after 24 h.
2. MMG buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| D-Mannitol (0.6 M) | 40 mM | 33.0 mL |
| MES (0.5 M) | 4 mM | 400 μL |
| MgCl2 (1.0 M) | 15 mM | 750 μL |
| Sterile ddH2O | n/a | 15.5 mL |
Add the reagents to a 50 mL Falcon tube and mix gently by inversion at room temperature. Avoid making bubbles. Make on the day of the experiment; do not save leftover buffer. Buffer should be stored at room temperature; it expires after 24 h.
3. W5 buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CaCl2 (1.0 M) | 125 mM | 6.25 mL |
| KCl (2.0 M) | 5.0 mM | 125 μL |
| MES (0.5 M, pH 5.7) | 0.5 mM | 40 μL |
| NaCl (1.0 M) | 1.54 mM | 77 μL |
| Sterile ddH2O | n/a | 43.5 mL |
Add the reagents to a 50 mL Falcon tube and mix gently by inversion at room temperature. Make on the day of the experiment; do not save leftover buffer. Buffer should be stored at room temperature; it expires after 24 h.
4. WI buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| D-Mannitol (0.6 M) | 500 mM | 41.67 mL |
| MES (0.5 M) | 4 mM | 400 μL |
| KCl (2.0 M) | 4 mM | 100 μL |
| Sterile ddH2O | n/a | 7.83 mL |
Add the reagents to a 50 mL Falcon tube and mix gently by inversion at room temperature. Make on the day of the experiment; do not save leftover buffer. Buffer should be stored at room temperature; it expires after 24 h.
5. PEG solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CaCl2 (1.0 M) | 100 mM | 500 μL |
| D-Mannitol (1.0 M) | 600 mM | 3.0 mL |
| PEG 4000 | 40% w/v | 2.0 g |
| Sterile ddH2O | n/a | Variable |
| Total | 5.0 mL |
Add the reagents to a 15 mL Falcon tube and place in a water bath at 55 °C. Allow for PEG to dissolve completely. Make on the day of the experiment; do not save leftover buffer. Buffer should be stored at room temperature; it expires after 24 h.
6. CTAB buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CTAB | 2% w/v | 2.0 g |
| EDTA (0.5 M, pH 8.0) | 20 mM | 4.0 mL |
| NaCl | 1.40 M | 8.2 g |
| Sterile ddH2O | n/a | Variable |
| Tris-HCl (1.0 M, pH 8.0) | 100 mM | |
| Total | 100.0 mL |
Add the reagents to a 100 mL Falcon tube and mix by inversion at room temperature until completely dissolved. Make on the day of the experiment; do not save leftover buffer. Buffer should be stored at room temperature; it expires after 24 h.
7. Enzyme suspension
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BSA (10%) | 0.0985% w/v | 100 μL |
| CaCl2 (1 M) | 4.93 mM | 50 μL |
| Cellulase | 2.9% w/v | 300 mg |
| Macerozyme | 0.69% w/v | 70 mg |
| Protoplast buffer (Recipe 1) | n/a | 10 mL |
Add cellulase, macerozyme, and protoplast buffer to a 50 mL Falcon tube. Incubate at 55 °C for 5 min. Mix by inversion halfway through incubation. Cool on ice for 5 min, then warm to room temperature for 5 min. Add CaCl2 and 10% BSA. Mix by inversion. This should be sufficient for 1–2 g of fresh leaf tissue. Make on the day of the experiment; do not save leftover buffer. It should be stored at room temperature; it expires after 24 h.
Laboratory supplies
1. FalconTM 50 mL high clarity conical centrifuge tubes (Fisher Scientific, catalog number 14-432-22)
2. Multi-fold towels (Uline, model number: S-13735)
3. Replacement blades (Havalon, model number: SSC60ADZ)
4. Falcon® 70 μm cell strainer (Fisher Scientific, catalog number: 08-771-2)
5. EppendorfTM safe-lock 2.0 mL microtube (Fisher Scientific, catalog number: 05-402-11)
6. EppendorfTM safe-lock 1.5 mL microtube (Fisher Scientific, catalog number: 05-402-27)
7. CorningTM CostarTM 12-well clear TC-treated multiple well plates, individually wrapped, sterile (Fisher Scientific, catalog number: 07-200-82)
8. Corporation bottles 250 mL glass, clear (Fisher Scientific, catalog number: 50-227-0179)
9. Wide-orifice 200 μL pipette tips (Fisher Scientific, catalog number: 50-101-668)
Equipment
1. Orbital shaker adjustable speed (Onilab, catalog number: SK-O330-M)
2. Refrigerated benchtop centrifuge (Avanti, model: J-15R)
3. Light microscope (Nikon, model: ECLIPSE Si)
4. Neubauer hemocytometer (Superior Marienfeld, catalog number: 0642010)
5. Vortex mixer (Onilab, model: MX-S)
Software and datasets
1. Cas-Analyzer (Seoul National University College of Medicine, 2016/11)
Procedure
文章信息
稿件历史记录
提交日期: Nov 22, 2025
接收日期: Dec 29, 2025
在线发布日期: Jan 15, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Higa, L. A., Bouwman, T. and Du, Z. (2026). Isolation and Transfection of Protoplasts From Maize Mesophyll Cells. Bio-protocol 16(3): e5596. DOI: 10.21769/BioProtoc.5596.
分类
植物科学 > 植物细胞生物学 > 细胞分离
生物科学 > 生物技术 > CRISPR/Cas9
植物科学 > 植物转化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












