- EN - English
- CN - 中文
Visual Nanoprobe-Enhanced Loop-Mediated Isothermal Amplification Protocol for Rapid Detection of Infectious Laryngotracheitis Virus from Avian Respiratory Swabs
基于可视化纳米探针增强的环介导等温扩增方法,用于禽类呼吸道拭子中传染性喉气管炎病毒的快速检测
(*contributed equally to this work) 发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5595 浏览次数: 126
评审: Antara SenguptaAnonymous reviewer(s)
Abstract
A prompt and accurate diagnosis of respiratory viral diseases in intensive poultry production is essential to safeguard animal health and ensure the economic sustainability of farms. Currently, much effort is being devoted to preventing the spread of the avian influenza virus in farms. However, the diagnosis of other relevant respiratory viruses, as infectious laryngotracheitis virus (ILTV), is also crucial. Indeed, infection by ILTV does lead to substantial economic losses due to high morbidity, reduced growth, and decreased productivity, making rapid detection a critical aspect of disease control. Conventional diagnostics, including PCR and qPCR, while sensitive and specific, require expensive laboratory infrastructure and well-trained personnel, limiting their deployment in field settings where immediate intervention is most valuable. To address these limitations, this protocol describes a portable molecular diagnostic workflow based on loop-mediated isothermal amplification (LAMP) combined with gold nanoparticle–DNA nanoprobes for specific and visual detection of ILTV directly at the point of need. Gold nanoparticles synthesized via the Turkevich method are functionalized with thiolated DNA probes, which undergo full-length, sequence-specific hybridization to LAMP amplicons, enabling a naked-eye colorimetric readout. The procedure integrates streamlined steps for DNA probe preparation, nanoparticle synthesis and assembly, and minimal sample processing, compatible with on-farm deployment. Results obtained with this workflow on field samples demonstrated 100% sensitivity and specificity, matching the performance of gold-standard assays. This approach offers a rapid, cost-effective, and equipment-free detection system of viral pathogens, enabling timely decision-making for disease containment and biosecurity. By overcoming the barriers of conventional diagnostics, this protocol enables producers with powerful tools for efficient monitoring and response to respiratory outbreaks in poultry farms.
Key features
• Direct ILTV detection in respiratory swabs in 35–45 min, bypassing DNA extraction with a rapid viral lysis step.
• Specific colorimetric readout via DNA nanoprobes with visual interpretation, requiring no specialized equipment or lab infrastructure.
• Achieves 100% sensitivity and specificity compared to qPCR, with a detection limit of 200 viral copies per reaction; validated in lab conditions with field samples.
• Modular design: Enables multiplex and customizable detection of other poultry pathogens, supporting rapid kit development and broad field application.
Keywords: Infectious laryngotracheitis virus (ILTV) (传染性喉气管炎病毒(ILTV))Graphical overview
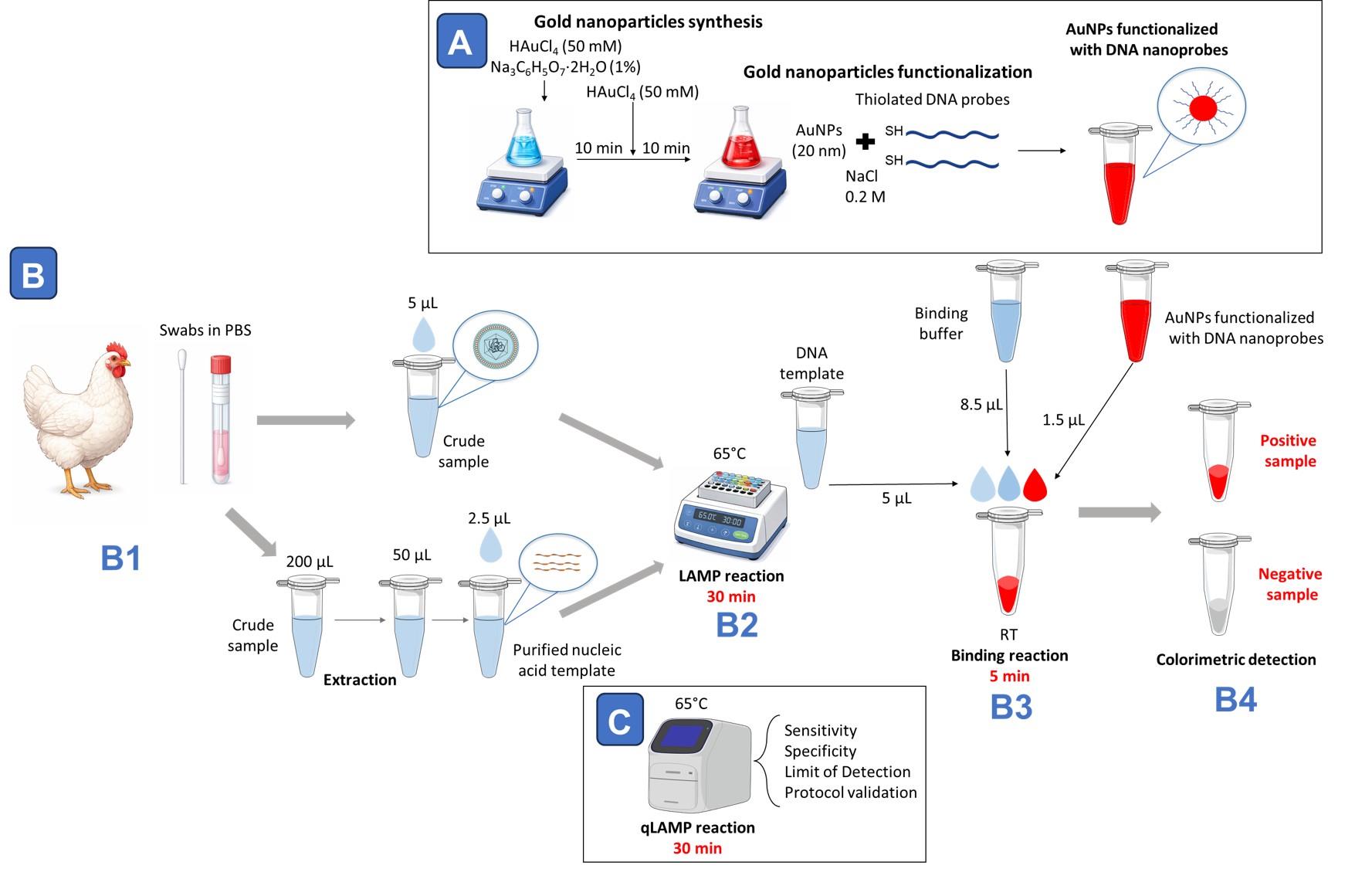
Workflow overview for avian respiratory virus detection combining loop-mediated isothermal amplification (LAMP) with AuNP-nanoprobe-based colorimetric readout. (A) For DNA-nanoprobe preparation, gold nanoparticles with a diameter of approximately 20 nm are synthesized using a two-step citrate reduction method and subsequently functionalized with thiolated DNA probes in the presence of NaCl. (B1) Oropharyngeal swabs collected in PBS are processed either as crude samples or following nucleic acid extraction. (B2) Samples are subjected to a 30-min LAMP reaction at 65 °C. (B3) After amplification, 5 μL of LAMP product is mixed with binding buffer (8.5 μL) and DNA nanoprobes (1.5 μL) and incubated at room temperature for 5 min. (B4) Hybridization with target DNA stabilizes nanoprobes in positive samples; in negative samples, absence of detection leads to nanoparticle destabilization and reaction clearing, enabling visual discrimination. (C) In parallel, qLAMP fluorescence reactions performed at 65 °C for 30 min are used to evaluate assay sensitivity, specificity, limit of detection, and overall protocol validation.
Background
Infectious laryngotracheitis (ILT) is a highly contagious respiratory disease that affects chickens, posing a significant economic threat to poultry production worldwide due to severe outbreaks characterized by high morbidity and variable mortality [1]. The etiological agent, infectious laryngotracheitis virus (ILTV), is a herpesvirus classified as Iltovirus gallidalpha 1, belonging to the Alphaherpesvirinae subfamily, with a double-stranded DNA genome of approximately 150 kb encoding around 80 predicted viral proteins. ILT manifests clinically in acute or subacute forms, with mortality rates up to 50% and characteristic symptoms including conjunctivitis, nasal discharge, respiratory distress, decreased egg production, and weight loss. These signs overlap with other avian respiratory infections such as infectious bronchitis virus (IBV) and avian metapneumovirus (aMPV), complicating diagnosis [2,3]. Additionally, ILTV establishes lifelong latency in trigeminal ganglia, rendering infected birds lifelong carriers and recurrent sources of infection, which further challenges control efforts in poultry farms.
Transmission occurs mainly through direct contact, and intensive farming conditions facilitate rapid spread. Early and accurate diagnosis of ILTV is crucial for timely intervention and outbreak containment to reduce economic losses in poultry production. Conventional diagnostic approaches have evolved from laborious and time-consuming laboratory-based methods to molecular detection techniques that include conventional PCR and real-time quantitative PCR (qPCR) [4]. Although highly sensitive and specific, these molecular methods require specialized equipment, well-trained personnel, and strict sample transport conditions, which limit their applicability for on-site, rapid diagnosis in field or low-resource settings [5].
Loop-mediated isothermal amplification (LAMP) has emerged as a promising alternative for point-of-care testing (POCT) due to its rapid amplification of nucleic acids at a constant temperature without the need for thermal cycling and its robustness against inhibitors commonly found in clinical samples. The LAMP reaction employs six primers recognizing eight distinct regions of the target DNA, enhancing specificity and amplification efficiency, which results in rapid and high-yield DNA amplification within less than 1 h [6]. Despite these advantages, visualizing LAMP amplicons reliably and specifically remains a challenge, as traditional methods such as pH indicators or turbidity assays can produce false positives due to nonspecific amplification [7].
To overcome these limitations, this study describes a novel diagnostic system that integrates LAMP targeting the ILTV glycoprotein E gene with a specific colorimetric detection method using DNA nanoprobes. The unique plasmonic properties of gold nanoparticles (AuNPs) enable naked-eye detection based on color change maintained by specific DNA–DNA hybridization, providing a highly specific and easy readout without complex fluorescence detection instruments or sophisticated laboratory infrastructure. The assay is compatible with both extracted nucleic acids and crude respiratory swab samples, the latter enabled by heat-induced lysis that eliminates the need for prior extraction and shortens the diagnostic turnaround to 35–45 min. The method achieves a detection limit of 200 viral copies per reaction, demonstrating 100% sensitivity and specificity compared to qPCR assays [8].
Moreover, the protocol is fully scalable and adaptable for development into diagnostic kits suitable for widespread use. Its modular primer design enables easy customization for detecting other poultry viral pathogens with minimal assay re-optimization. Furthermore, this platform supports multiplex detection, allowing simultaneous identification of multiple viral pathogens in a single reaction tube, facilitating comprehensive field monitoring with streamlined workflows and rapid visual readouts.
In summary, this LAMP-nanoprobe system represents an advance in rapid, cost-effective, point-of-care diagnostics for ILTV and other infectious agents, enhancing timely disease management and biosecurity in poultry production worldwide.
Materials and reagents
Biological materials
1. Oropharyngeal swabs from chickens and turkeys positive for infectious laryngotracheitis virus (ILTV) resuspended in 1 mL of 1× PBS (crude samples), kindly provided by the Centro de Sanidad Avícola de Cataluña y Aragón (CESAC)
Reagents
For sample collection, nucleic acid extraction, and LAMP amplification/visualization:
1. Swabs for oropharyngeal sample collection (Merck, catalog number: WHAWB100032)
2. MagMAX Viral/Pathogen I Nucleic Acid Isolation kit (Invitrogen, catalog number: A48310)
3. DNA Zap for degradation solution (Invitrogen, catalog number: AM9890)
4. WarmStart® LAMP kit (DNA & RNA) (New England Biolabs, catalog number: E1700)
5. LAMP fluorescent dye (New England Biolabs, catalog number: B1700)
6. Nuclease-free water (Invitrogen, catalog number: 10977023)
7. Primers targeting ILTV glycoprotein E (gE) gene (Macrogen-Inc., South Korea, custom synthesis)
8. Agarose (Bio-Rad Laboratories, catalog number: 4561094)
9. TAE 50× for molecular biology (Condalab, catalog number: A4686-1000)
10. GelRedTM nucleic acid gel stain (Biotium Inc., catalog number: 41003)
11. 6× DNA loading buffer (TaKaRa, catalog number: 9156)
12. NZYDNA ladder VI, 50–1,500 bp (NZYtech, catalog number: MB08903)
For AuNPs synthesis:
1. Chloroauric acid (HAuCl4·3H2O) (Sigma-Aldrich, catalog number: 254169)
2. Trisodium citrate dihydrate (Na3C6H5O7·2H2O) (Sigma-Aldrich, catalog number: 71370)
For AuNPs functionalization:
1. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 7647-14-5)
2. PBS 1× (Condalab, catalog number: 4015)
3. DNA oligonucleotide nanoprobes (Macrogen-Inc., South Korea, customized synthesis, 5’-thiol modification for nanoprobe functionalization)
4. Magnesium chloride (MgCl2), 100 mM solution (Sigma-Aldrich, catalog number: 208337)
5. Tween-20 (polyoxyethylene sorbitan monolaurate) (Sigma-Aldrich, catalog number: P9416)
6. Sodium acetate (CH3COONa) (Sigma-Aldrich, catalog number: S2889)
7. Dithiothreitol (DTT) (Sigma-Aldrich, catalog number D0632)
8. Ethanol absolute ≥99.8% (VWR, catalog number: 20821.296)
For plasmid construction:
1. One ShotTM LB agar plates with antibiotics (Sigma-Aldrich, catalog number: A55802)
2. X-Gal (NZYtech, catalog number: MB025)
3. Dimethylformamide (Sigma-Aldrich, catalog number: 493074)
4. Kit TOPOTM TA CloningTM (Thermo Fisher Scientific, catalog number: K4575-J10)
5. MaximeTM PCR PreMix i-Taq (iNtRON Biotechnology DR, catalog number: 25025)
6. NZYSpeedy Miniprep (NZYtech, catalog number: MB21001)
Solutions
1. Binding buffer (see Recipes)
Recipes
1. Binding buffer
| Reagent | Stock concentration | Final concentration |
| NaCl | 5 M | 2 M |
| MgCl2 | 1 M | 80 mM |
| Tween-20 | 20% | 0.03% |
| Tris-HCl, pH 7.5 | 150 mM | 25 mM |
Mix all components to prepare 150 μL of a stock solution.
Laboratory supplies
1. 10 μL pipette tips (Eppendorf, catalog number: 0030000811)
2. 200 μL pipette tips (Eppendorf, catalog number: 0030000889)
3. 1,000 μL pipette tips (Eppendorf, catalog number: 0030000927)
4. 10 μL pipette filter tips (VWR Avantor, catalog number: 76322-132)
5. 200 μL pipette filter tips (VWR Avantor, catalog number: 76322-150)
6. 1,000 μL pipette filter tips (VWR Avantor, catalog number: 76322-154)
7. Microcentrifuge Eppendorf tubes 3810× 1,5 mL (Eppendorf, catalog number: 0030125215)
8. Real-time PCR tubes, 0.2 mL, in strips (Deltalab, catalog number: 4094.5BP)
9. Erlenmeyer flask 50 mL (Fisher Scientific, catalog number: 10389789)
10. Polycarbonate bottle 1,000 mL (DDBiolab, catalog number: 360516)
11. WHEATON® liquid scintillation 20 mL vial with attached cap (Merck, catalog number: DWK986540-500EA)
12. Petri dish (VWR Avantor, catalog number: 22PS4876)
13. Magnetic stir bar (Sigma Aldrich, catalog number: 41122401)
Equipment
1. Mastercycler® nexus GX2 thermocycler (Eppendorf, catalog number: 6336000023)
2. QuantStudioTM 5 Real-Time PCR System (Thermo Fisher Scientific, catalog number: A47327)
3. Thermomixer C (Eppendorf, catalog number: 5382000015)
4. NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, catalog number: ND-2000)
5. Qubit 4 fluorometer (Thermo Fisher Scientific, catalog number: Q33238)
6. Minispin Plus (Eppendorf, catalog number: 5453000015)
7. Magnetic stirrer with heating up to 340 °C (Labbox, catalog number: STIH-020-001)
8. Microcentrifuge 5427 R (Eppendorf, catalog number: 5429000010)
9. E-BOX CX5 (VWR avantor, 733-2832)
10. Wide Mini-Sub Cell GT Horizontal Electrophoresis System, 15 × 7 cm tray, with casting gates (Bio-Rad, catalog number:1704405)
11. KingFisher Flex robot (Thermo Fisher Scientific, catalog number: 5400610)
12. Savant SpeedVac DNA 130 vacuum concentrator (Thermo Fisher Scientific, catalog number: DNA130-230)
13. Telstar Bio II advance plus (Telstar, catalog number: 528287)
14. Telstar V-100 (Telstar, catalog number: 23001)
15. Crop oven, Hareus 7000 series (Vidra Foc, catalog number: TPPP.50042301)
16. Rotator for Eppendorf tubes (Ovan Laboratory equipment, catalog number: 20461000001013)
17. Eppendorf Research plus 10 μL pipette (Eppendorf, catalog number: 3123000020)
18. Eppendorf Research plus 20 μL pipette (Eppendorf, catalog number: 3123000039)
19. Eppendorf Research plus 200 μL pipette (Eppendorf, catalog number: 3123000055)
20. Eppendorf Research plus1000 μL pipette (Eppendorf, catalog number: 3123000063)
21. Racks
22. Liquid nitrogen (N2) tank
23. Freezer (-20 °C)
24. Refrigerator (2–8 °C)
Software and datasets
1. GenBank (https://www.ncbi.nlm.nih.gov/nuccore, 7/June/2024)
2. BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi, 7/June/2024)
3. Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/, 7/June/2024)
4. PrimerExplorer V5 (https://primerexplorer.jp/e/, 10/June/2024)
5. RNAfold WebServer (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi, 4/July/2024)
6. SnapGene (version 7.1.2, released 28 February 2024); requires a license
7. Design and Analysis Software (version 2.8, released 18 January 2024); requires a license
Procedure
文章信息
稿件历史记录
提交日期: Nov 17, 2025
接收日期: Dec 28, 2025
在线发布日期: Jan 15, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Cea-Callejo, P., Trenado, C., Doménech, A., Madrid, R. and Benítez, L. (2026). Visual Nanoprobe-Enhanced Loop-Mediated Isothermal Amplification Protocol for Rapid Detection of Infectious Laryngotracheitis Virus from Avian Respiratory Swabs. Bio-protocol 16(3): e5595. DOI: 10.21769/BioProtoc.5595.
分类
微生物学 > 病原体检测 > RT-LAMP
分子生物学 > 纳米颗粒 > 传感器
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link