- EN - English
- CN - 中文
Advancing EAE Modeling: Establishment of a Non-Pertussis Immunization Protocol for Multiple Sclerosis
推进 EAE 动物模型研究:一种无需百日咳毒素的多发性硬化免疫建模方案的建立
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5589 浏览次数: 28
评审: Anonymous reviewer(s)

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1641 阅读
Abstract
Experimental autoimmune encephalomyelitis (EAE) is a widely used rodent model of multiple sclerosis (MS), typically induced with pertussis toxin (PTX) to achieve robust disease onset. However, PTX has been shown to exert broad immunomodulatory effects that include disruption of G protein-coupled receptor (GPCR) signaling, altered T-cell response, and exogenous suppression of regulatory T cells, all of which are not present in human MS pathophysiology. Moreover, PTX also obscures the sex differences observed in MS, limiting the translational value of EAE models that rely on it. Given EAE’s widespread use in preclinical therapeutic testing, there is a critical need for a model that better recapitulates both clinical and immunological features of MS without PTX-induced confounds. Here, we demonstrate a non-pertussis toxin (non-PTX) EAE model in C57BL/6 mice, using optimized concentrations of complete Freund’s adjuvant (CFA), Mycobacterium tuberculosis, and myelin oligodendrocyte glycoprotein (MOG35-55) peptide. This model recapitulates hallmark features of MS that include demyelination, neuroinflammation, motor deficits, and neuropathic pain. Importantly, it retains sex-specific differences in disease onset and pathology, providing a more physiologically and clinically relevant platform for mechanistic and translational MS research.
Key features
• Establishes a reproducible clinically relevant EAE protocol in C57BL/6 mice that induces MS-like neurological deficits without pertussis toxin.
• Recapitulates hallmark MS pathology, including neuroinflammation, demyelination, axonal injury, and neuropathic pain.
• Eliminates the off-target effects of pertussis toxin, which confound GPCR-mediated signaling, T-cell responses, and neuroimmune interactions.
Keywords: Pertussis toxin (PTX) (百日咳毒素)Graphical overview
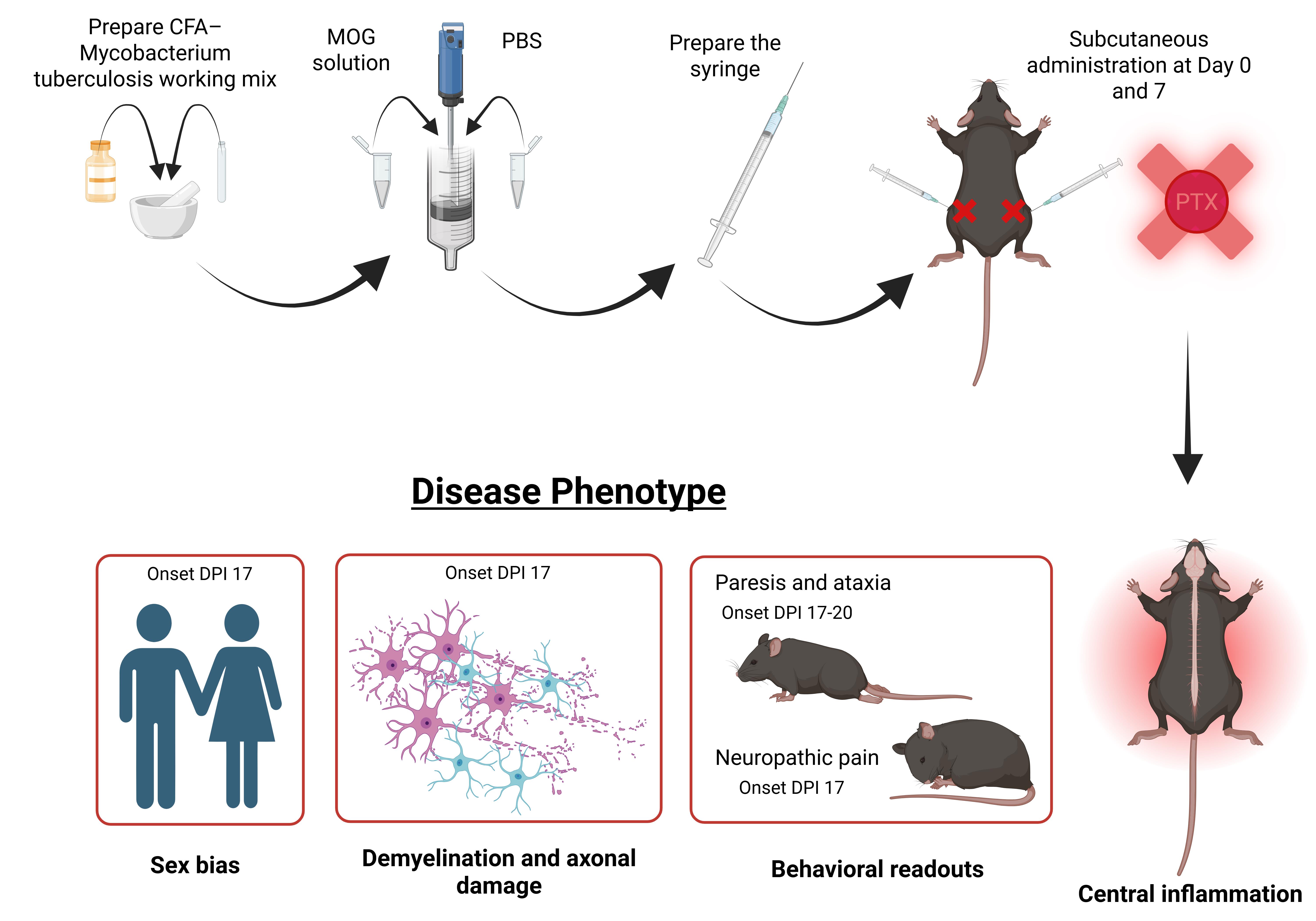
Graphical overview of experimental autoimmune encephalomyelitis (EAE) immunization and disease phenotype manifestation without pertussis toxin. DPI: Days post immunization.
Background
Multiple sclerosis (MS) is an autoimmune disorder with over 8 million individuals diagnosed [1]. Current treatments rely on immunosuppressive disease modifying therapies; however, these therapies have limited therapeutic efficacy and significant side effects [2–4].
Experimental autoimmune encephalomyelitis (EAE) is a rodent model of MS extensively used in the field to investigate disease pathophysiology and advance therapeutic discovery and curative strategies [5–9]. EAE mimics key MS pathophysiology and successfully recapitulates the hallmarks of the disease—demyelination, neuroinflammation, gliosis, and axonal damage [6,9–12]. Moreover, it also replicates the two prominent symptoms of MS—neuropathic pain and motor deficits [13–15]. Most often, active EAE induction in C57BL/6 mice requires immunization with the myelin oligodendrocyte glycoprotein (MOG35-55) peptide emulsified in complete Freund’s adjuvant (CFA) and heat-killed Mycobacterium tuberculosis, alongside co-administration of pertussis toxin (PTX) [6,7,16].
PTX is a bacterial exotoxin that facilitates disease induction by artificially disrupting the blood–brain barrier (BBB), enabling CNS infiltration of peripheral immune cells [17,18]. However, apart from its synthetic effect on immune cell infiltration in the CNS, it also introduces several confounding variables. PTX disrupts G protein-coupled receptor (GPCR) signaling through ADP-ribosylation, complicating studies that seek to understand GPCR function in neuroinflammation and immune regulation, which is relevant given the growing recognition of GPCRs in MS pathogenesis [19–22].
PTX’s broad immunomodulatory properties extend beyond adjuvant effects. It alters the immune landscape through suppression of regulatory T cells and skews T helper responses toward pathogenic Th1 and Th17 phenotypes, which artificially exacerbates the pro-inflammatory effect of EAE pathology [23–26]. Additionally, it has also been shown to mask sex differences that are prevalent in MS pathology [27]. Together, these factors limit the translational relevance of PTX-based EAE models and warrant the use of alternative protocols that better preserve physiological immune responses.
Recent work from our lab demonstrates that EAE can be reliably induced in C57BL/6 mice without PTX, using optimized antigen and adjuvant conditions [27–31]. This non-PTX EAE model recapitulates key clinical and pathological features of MS, including motor deficits, neuropathic pain, demyelination, neuroinflammation, gliosis, and axonal loss, without relying on artificial BBB disruption. Importantly, omitting PTX reveals biologically meaningful sex-specific differences in disease onset, immune activation, and neurodegeneration that are often masked in traditional PTX-dependent EAE models [27,31].
Increase in myelin and axonal pathology, immune cell infiltration, and glial activation in the brain and lumbar spinal cord have been assessed in our model using immunohistochemistry, western blotting, and flow cytometry [27,29,30]. Moreover, in our non-PTX EAE model, females develop paresis significantly earlier than males; however, immunopathological analyses revealed heightened glial and immune activation in EAE males, mirroring the clinical trajectory of MS. Specifically, male EAE animals exhibited significantly greater CD45+ leukocyte and peripheral macrophage infiltration in the lumbar spinal cord relative to female EAE mice [27].
Given the increasing interest in modeling MS pathophysiology in a more biologically and translationally relevant manner, the non-PTX EAE model offers a powerful platform for mechanistic studies, therapeutic testing, and the investigation of sex as a biological variable. In this paper, we describe a detailed and reproducible protocol for non-PTX EAE induction in C57BL/6 mice, along with phenotypic readouts and histopathological assessments that validate the model’s utility in capturing the heterogeneity of MS.
Materials and reagents
Biological materials
1. Heat-inactivated Mycobacterium tuberculosis H37Ra (Fisher Scientific, catalog number: DF3114-33-8), stored at -20 °C
2. C57BL6/J mice (Jackson Laboratories, stock number: 000664)
Laboratory supplies
1. MOG35–55 peptide (Biosynthesis, catalog number: 12668-10)
2. Complete Freund’s Adjuvant (CFA) (InvivoGen, catalog number: vac-cfa-10)
3. 1× Dulbecco's phosphate-buffered saline (DPBS), no calcium, no magnesium (Gibco, catalog number: 14190144), pH 7.0–7.3
4. 10 and 1 mL syringes (without needles) (BD, catalog numbers: 309605 and 309628)
5. 20 G needles (precision glide hypodermic injection needle, short bevel 20 G × 1") (BD, catalog number: 305178)
6. 50 mL conical tubes (50 mL high clarity conical centrifuge tubes) (Falcon, catalog number: 14-432-22)
7. Parafilm (Thomas Scientific, catalog number: 1222J99)
8. Isoflurane (Kent Scientific, catalog number ISO-6)
Equipment
1. Small mortar and pestle (porcelain mortar and pestle sets, deep form) (Thomas Scientific, catalog number: 1201U67)
2. Tissue homogenizer (Biospec Products, catalog number: 985370-04)
3. Standard laboratory chemical fume hood
Procedure
文章信息
稿件历史记录
提交日期: Oct 23, 2025
接收日期: Dec 23, 2025
在线发布日期: Feb 2, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Gupta, S., Arnab, S., Stehle, E. and Nguyen, K. L. (2026). Advancing EAE Modeling: Establishment of a Non-Pertussis Immunization Protocol for Multiple Sclerosis. Bio-protocol 16(3): e5589. DOI: 10.21769/BioProtoc.5589.
分类
免疫学 > 炎症性疾病 > 多发性硬化
免疫学 > 动物模型 > 小鼠
神经科学 > 神经系统疾病 > 动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link