- EN - English
- CN - 中文
An Ex Vivo Lung Histoculture Model for Studying Pulmonary Infection and Immune Response With SARS-CoV-2 as an Example of RNA Virus
用于研究肺部感染与免疫反应的离体肺组织培养模型,以 SARS-CoV-2 为 RNA 病毒研究示例
(*contributed equally to this work) 发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5552 浏览次数: 751
评审: David PaulAnonymous reviewer(s)

相关实验方案

冷冻保存的人类全肠组织免疫细胞分离、类器官构建及共培养建立方案
Enrique Gamero-Estevez [...] Martin Resnik-Docampo
2025年01月05日 3727 阅读
Abstract
The tissue explant culture (histoculture) is a method that involves maintaining small pieces taken from an organ ex vivo or post mortem in a controlled laboratory setting. Such a technique has a number of advantages: unlike the 2D, organoid, or on-chip cultures, tissue explants preserve the whole complexity of the original tissue in vivo, its structure, extracellular matrix, and the diverse cell populations, including resident immune cells. The explant culture method can be applied to human tissue specimens obtained from biopsies or autopsies, provided that proper ethical protocols are followed. This avoids the difficulties that may arise in translating results obtained on animal models into biomedical research for humans. This advantage makes histocultures especially desirable for studying human pathogenesis in the course of infectious diseases. The disadvantage of the method is the limited lifespan of the cultured tissues; however, a number of approaches allow extending tissue viability to a period sufficient for observing the infection onset and development. Here, we provide a protocol for lung explant maintenance that allows tracing the local effects of infection with SARS-CoV-2 in humans. Further applications of the lung tissues cultured according to this protocol include, but are not limited to, histochemical and immunohistochemical studies and microscopy, FACS, qPCR, and ELISA-based analysis of the conditioned culture media.
Key features
• The protocol relies on lung tissue culture on collagen rafts at the air–liquid interface, followed by infection with viral agents.
• The developed system provides a laboratory-controlled model to investigate the mechanisms of SARS-CoV-2 infection and allows further histological/immunohistochemical, qPCR, FACS, and xMAP cytokine analysis.
• Successful establishment of explant culture requires basic cell culture experience. Successful viral infection requires access to a BSL3 laboratory and relevantly trained personnel.
Keywords: Ex vivo model (离体模型)Graphical overview
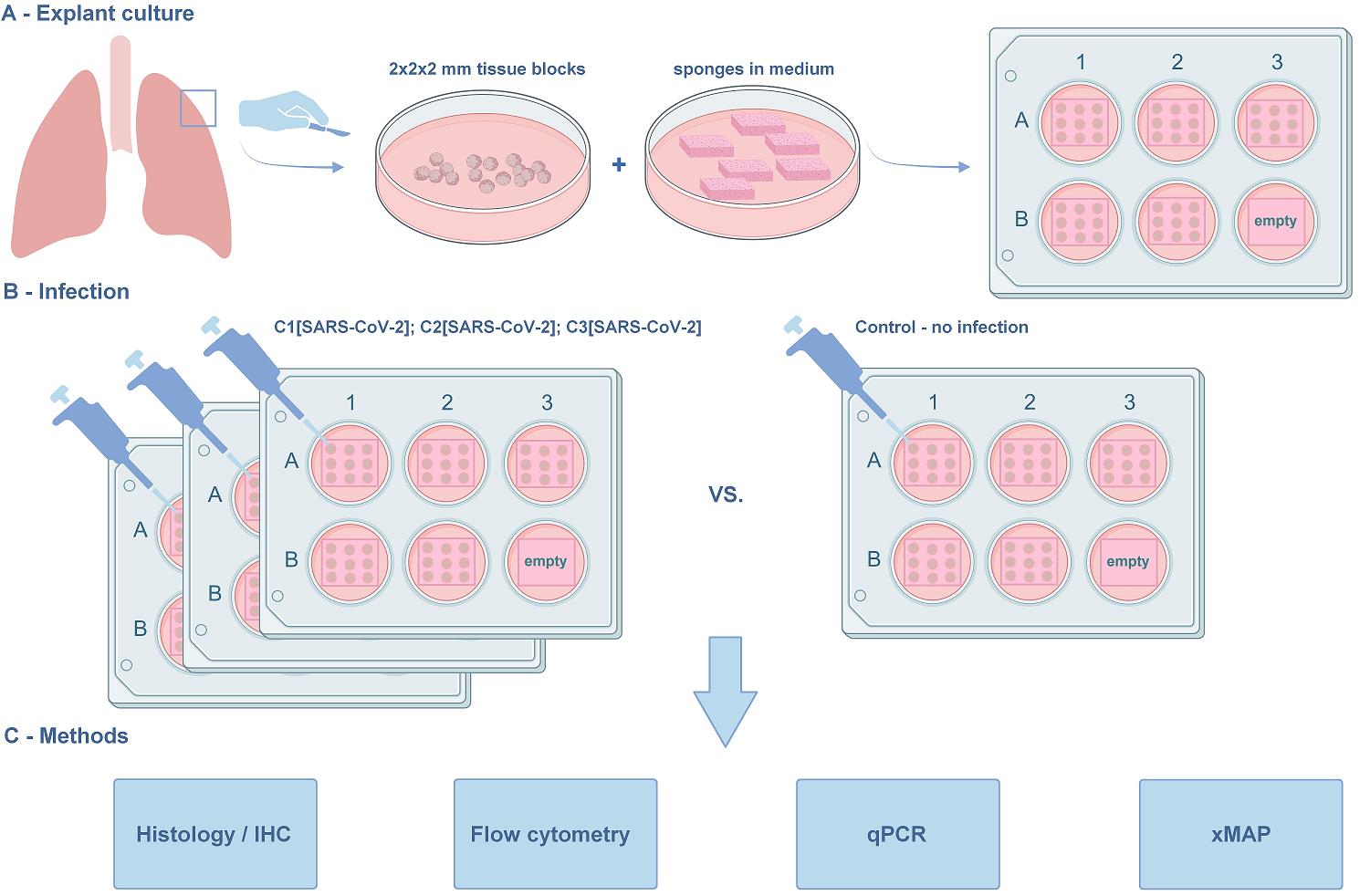
The lung explant culture. (A) Establishment of the lung explant culture at the air–liquid interface. The blocks are derived from the marginal lung tissue of patients with carcinoma undergoing lobe resection. The macroscopically and microscopically intact lung tissue, as assessed by a pathologist, is used in the protocol. (B) Viral infection. The model is suitable for studying SARS-CoV-2 infection and in situ immune response. Three concentrations of the virus were used along with the non-infected negative control. (C) Methods for further analysis compatible with the histoculture model. The protocol was used in [1]. The graphical overview was created with BioRender.com.
Background
The lung is an organ with complicated architecture and diverse cell content that comprises over 40 cell types [2]. For a long time, “the big five” lung diseases—asthma, chronic obstructive pulmonary disease (COPD), acute lower respiratory tract infections, lung cancer, and tuberculosis (TB)—have been among the most common causes of illness, disability, and death worldwide. The recent COVID-19 pandemic has added significantly to the number of respiratory disease cases and associated mortality [3]. This explains the need for meticulously searching for the most relevant, easy-to-maintain, and cost-effective lung models to study lung disease pathogenesis and to test therapeutic strategies under controlled laboratory conditions.
The available lung models include 2D cell cultures of either transformed or non-transformed cell lines. The transformed cell lines, most often cancerous, are easy to maintain and show higher reproducibility. Apart from cancer research, these have been used to investigate other lung pathologies, including infection [4–6]. Despite their relative simplicity, they are limited in use due to the lack of original tissue architecture, cell matrix, and population diversity. Primary non-transformed cells have better potential to imitate in vivo infection [7]. However, they too do not faithfully reflect the complex lung architecture. Hence, attempts have been made to design 3D tissue models, for example, to co-culture cells [8] from 2D monocultures and grow them in 3D [9].
3D models of the lung include organoids and lung-on-a-chip assays. Organoids are 3D culture systems derived from adult/fetal/induced pluripotent stem cells that can proliferate and differentiate into structures that, in part, mimic the organ. Although lung organoids are achieving increased complexity, they remain restricted in both cellular diversity and the sophistication of their extracellular matrix composition [10]. The same is true for the micro-engineered lung-on-a-chip models [11].
Animal models also have their drawbacks. Often, they do not fully represent the human lung pathology due to histological and physiological differences of the lung, including its immune microenvironment [12–14]. Mouse humanization or other additional procedures are often required to study human diseases, which makes such an approach costly and difficult [15,16].
The lung explant culture (histoculture) is a method of maintaining 3D blocks of lung tissue in the laboratory [17]. The tissue architecture, cell diversity, and intercellular interactions are thus largely preserved. Thus, histoculture models show promise for a number of applications in biomedicine and basic research [18].
To date, lung histocultures have been successfully implicated in lung development studies [19,20]. In cancer research, explants from the intact and tumor lung areas were used in a study of metabolic alterations associated with malignancy [21] and to evaluate the effects of therapeutic compounds on cell metabolism and cell death [22]. Respiratory infection studies also greatly benefit from this model [23].
Due to the consistently high relevance of this topic, here, we address a pulmonary infection model. As the lung has a barrier function in the organism, it is a frequent site of diverse infections and associated inflammation and tissue damage [24]. A significant part of the pathogens are RNA viruses, including influenza viruses, respiratory syncytial virus (RSV), and, more recently, some pneumonia-causing coronaviruses. Indeed, SARS-CoV-2 presented the most recent instance of a pathogen with high lung tropism [25,26]; the COVID-19 pandemic fueled a number of new studies of pneumonia pathogenesis [26–29]. Similar to work done on influenza viruses [23], we propose a model of controlled lung infection, test several virus assessment methods, and prove the compatibility of the model with diverse methods to further analyze tissue damage and immune response.
The limited viability of lung tissue presents the greatest difficulty and has to be addressed carefully in the experimental setup. Here, we adapted our previous protocols [30,31,38], developed for different tissue types, to the lung. We report a lung histoculture model that maintains tissue morphology and viability for 10 days—a sufficient time to trace the infection of SARS-CoV-2 in situ. We believe that, similar to what was done on other models, lung histocultures may be used for co-infection studies [32,33] and drug testing [34–36].
Materials and reagents
Biological materials
1. Virus strain: SARS-CoV-2 B.1.1.1 variant (GISAID EPI_ISL_421275), deposited at the National Research Center for Epidemiology and Microbiology Named After Honorary Academician N.F. Gamaleya.
Caution: All experiments using infectious SARS-CoV-2 should be performed in a biosafety level 3 (BSL3) laboratory.
2. Cell line: Vero C1008 clone E6 (ATCC, catalog number: CRL-1586TM)
3. Lung tissue: obtained from the post-surgical material of individuals with primary lung carcinoma who underwent lobectomy. None of the individuals received therapy prior to surgery. The protocol was approved by the Moscow City Ethics Committee and conducted in accordance with the local legislation and institutional requirements.
Reagents
1. PBS (10×), pH 7.2 (GibcoTM, Thermo Fisher Scientific, catalog number: 70013032); store at room temperature (RT)
2. Gelfoam® hemostatic agent absorbable gelatin topical sponge (Pfizer, catalog number: 00009031508); store at RT
3. RPMI-1640 (GibcoTM, Thermo Fisher Scientific, catalog number: 11875093); store at RT
4. Anti-Anti (100×) antibiotic-antimycotic (GibcoTM, Thermo Fisher Scientific, catalog number: 15240-062); store at 4 °C
5. FBS HyClone (HyCloneTM, Cytiva, catalog number: SH30071.03); store at 4 °C
6. GlutaMAX (GibcoTM, Thermo Fisher Scientific, catalog number: 35050087); store at 4 °C
7. Non-essential amino acids (GibcoTM, Thermo Fisher Scientific, catalog number: 11140050); store at 4 °C
8. Sodium pyruvate (GibcoTM, Thermo Fisher Scientific, catalog number: 11360070); store at 4 °C
9. RNAlater (RNA stabilization reagent) (InvitrogenTM, Thermo Fisher Scientific, catalog number: AM7020); store at RT
10. Paraformaldehyde (PFA) (Sigma-Aldrich, Merck Life Science LLC, catalog number: 158127); store at RT in a dark place
11. Collagenase, Type IV, powder (GibcoTM, Thermo Fisher Scientific, catalog number: 17104019); store at 4 °C; protect from light
12. DNase I (InvitrogenTM, Thermo Fisher Scientific, catalog number: 18047019); store at -20 °C
13. Live-Dead fixable stain AlexaFluor 350 (InvitrogenTM, Thermo Fisher Scientific, catalog number: L23105); store at 4 °C; protect from light
14. Human BD Fc BlockTM (BD PharmingenTM, catalog number: 564219); store at 4 °C
15. Stain buffer (FBS) (BD PharmingenTM, catalog number: 554656); store at 4 °C
16. APC-R700 mouse anti-human CD45 (BD PharmingenTM, catalog number: 566041); store at 4 °C; protect from light
17. BD HorizonTM BV510 mouse anti-human CD3 (BD PharmingenTM, catalog number: 566779); store at 4 °C; protect from light
18. BD HorizonTM BUV661 mouse anti-human CD4 (BD PharmingenTM, catalog number: 569782); store at 4 °C; protect from light
19. BD HorizonTM BUV395 mouse anti-human CD8 (BD PharmingenTM, catalog number: 569178); store at 4 °C; protect from light
20.BD HorizonTM BUV805 mouse anti-human CD14 (BD PharmingenTM, catalog number: 612902); store at 4 °C; protect from light
21. BD HorizonTM BUV737 mouse anti-human CD16 (BD PharmingenTM, catalog number: 612786); store at 4 °C; protect from light
22. BD HorizonTM BUV496 mouse anti-human NCAM-1 (CD56) (BD PharmingenTM, catalog number: 569467); store at 4 °C; protect from light
23. BDTM PE-CyTM 7 mouse anti-human CD11c (BD PharmingenTM, catalog number: 652358); store at 4 °C; protect from light
24. BDTM PE mouse anti-human CD123 (BD PharmingenTM, catalog number: 340545); store at 4 °C; protect from light
25. BDTM APC-CyTM 7 mouse anti-human HLA-DR (BD PharmingenTM, catalog number: 335796); store at 4 °C; protect from light
26. Alexa Fluor® 647 mouse anti-human CD66b (BD PharmingenTM, catalog number: 561645); store at 4 °C; protect from light
27. Ethanol 96%, EMPROVE® EXPERT, Ph. Eur., ChP, JP, USP (Millipore, Merck Life Science LLC, catalog number: 100967); store at RT
Caution: This chemical is flammable.
28. Toluene (Sigma-Aldrich, Merck Life Science LLC, catalog number: 589578100); store at RT
Caution: This chemical is flammable.
29. Paraffin HISTOMIX (BioVitrum, catalog number: 247/NS); store at RT
30. Eosin solution (BioVitrum, catalog number: HK-ES-B250); store at RT
31. Hematoxylin solution (BioVitrum, catalog number: HK-G0-CD05); store at RT
32. Anti-N protein antibodies (HyTest, catalog number: C706, rabbit monoclonal); store at 4 °C
33. 0.1% TritonX-100 (Sigma-Aldrich, Merck Life Science LLC, catalog number: X100-100ML); store at RT
Caution: This chemical is toxic.
34. Dual endogenous enzyme block (Dako, Agilent, catalog number: S200380-2); store at 4 °C
Caution: This chemical is toxic.
35. UltraVision Detection HRP DAB kit (LabVision Corp., Thermo Fisher Scientific, catalog number: TL-060-QHD); store at 4 °C
36. Anti-CD68 antibodies (Talent Biomedical, catalog number: AR0349); store at 4 °C
37. Shandon-Mount medium (EprediaTM, Thermo Fisher Scientific, catalog number: 1900333); store at RT
Caution: This chemical is toxic and flammable.
38. RLT buffer (QIAGEN, catalog number: 79216); store at RT
Caution: This chemical is toxic.
39. β-mercaptoethanol (Calbiochem®, Merck Life Science LLC, catalog number: 444203); store at RT
Caution: This chemical is toxic and flammable.
40. RNeasy Mini kit (QIAGEN, catalog number: 74104); store at RT
Caution: Contains chemicals not suitable for use with bleach.
41. RIBO-prep kit (AmpliSens®, catalog number: K2-9-Et-100); store at 4 °C
42. OneTube RT-PCRmix (Eurogene, catalog number: SK101M); store at -20 °C
43. MILLIPLEX MAP human cytokine/chemokine magnetic bead panel (Millipore, Merck Life Science LLC, catalog number: HCYTMAG-60K-PX41); store at 4 °C
44. Tween-20 (Sigma-Aldrich, catalog number: P9416); store at RT
45. Luminex sheath fluid (Luminex, catalog number: 40-50000); store at RT
Solutions
1. Transportation medium (see Recipes)
2. Complete culture medium (see Recipes)
3. Virus expansion medium (see Recipes)
4. PBS 1× (see Recipes)
5. PFA 4% (see Recipes)
6. Digestion mix (see Recipes)
7. Wash buffer for automatic wash (see Recipes)
8. PCR primer-probe mix for viral production in medium testing (see Recipes)
9. PCR primer-probe mix for tissue viral load testing (see Recipes)
Recipes
1. Transportation medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI-1640 | 99% | 495 mL |
| Anti-anti (100×) | 1% | 5 mL |
| Total | 100% | 500 mL |
Should be prepared in aseptic conditions in the laminar flow cabinet. Store at 4 °C.
2. Complete culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI-1640 | 81% | 405 mL |
| FBS HyClone heat-inactivated | 15% | 75 mL |
| GlutaMAX (100×) | 1% | 5 mL |
| Non-essential amino acids (100×) | 1% | 5 mL |
| Sodium pyruvate (100 mM) | 1% | 5 mL |
| Anti-anti (100×) | 1% | 5 mL |
| Total | 100% | 500 mL |
Should be prepared in aseptic conditions in the laminar flow cabinet. FBS should be heat-inactivated in a water bath at 56 °C for 40 min. After mixing, gently pipette the complete culture medium. Prewarm it at 37 °C in a water bath before starting work. Store at 4 °C.
3. Virus expansion medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM | 94% | 480 mL |
| FBS HyClone heat-inactivated | 2% | 10 mL |
| GlutaMAX (100×) | 1% | 5 mL |
| Anti-anti (100×) | 1% | 5 mL |
| Total | 100% | 500 mL |
Should be prepared in aseptic conditions in the laminar flow cabinet. FBS should be heat-inactivated in a water bath at 56 °C for 40 min. After mixing, gently pipette the complete culture medium. Prewarm it at 37 °C in a water bath before starting work. Store at 4 °C.
4. PBS 1×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBS (10×) pH 7.2 | 10% | 100 mL |
| MQ | 90% | 900 mL |
| Total | 100% | 1,000 mL |
Should be prepared in aseptic conditions in the laminar flow cabinet. Store at RT.
5. PFA 4% (m/v)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PFA 95% powder | 4% | 4 g |
| PBS 1× | 96% | 100 mL |
| Total | 100% | 100 mL |
Dissolve 4 g of PFA in 90 mL of PBS 1× at 60 °C. Let it cool to RT and adjust the volume to 100 mL with PBS 1×. Store at RT.
6. Digestion mix
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Collagenase, Type IV | 5 mg/mL | 125 μL |
| DNase I, bovine | 40 U/mL | 5 μL |
| RPMI-1640 | 870 μL | |
| Total | 1 mL |
Store at 4 °C.
7. Wash buffer for automatic wash
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tween-20 | 0.05% | 1 mL |
| PBS 1× | 99.95% | 1,999 mL |
| Total | 100% | 20,00 mL |
Store at 4 °C.
8. PCR primer-probe mix for viral production in medium testing (per 1 reaction)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
Forward primer N2 5’-TTACAAACATTGGCCGCAAA-3’ | 500 nM | 0.5 μL |
Reverse primer N2 5’-GCGCGACATTCCGAAGAA-3’ | 500 nM | 0.5 μL |
Probe N2 FAM5’-ACAATTTGCCCCCAGCGCTTCAG-3’BHQ1 | 500 nM | 0.5 μL |
Forward primer N3 5’-GGGAGCCTTGAATACACCAAAA-3’ | 500 nM | 0.5 μL |
Reverse primer N3 5’-TGTAGCACGATTGCAGCATTG -3’ | 500 nM | 0.5 μL |
Probe N3 VIC5’-ATCACATTGGCACCCGCAATCCTG-3’BHQ2 | 500 nM | 0.5 μL |
| ddH2O | 2 μL | |
| Total | 5 μL |
Store at -20 °C.
9. PCR primer-probe mix for tissue viral load testing (per 1 reaction)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
Forward primer N2 5’-TTACAAACATTGGCCGCAAA-3’ | 500 nM | 0.5 μL |
Reverse primer N2 5’-GCGCGACATTCCGAAGAA-3’ | 500 nM | 0.5 μL |
Probe N2 FAM5’-ACAATTTGCCCCCAGCGCTTCAG-3’BHQ1 | 500 nM | 0.5 μL |
Forward primer N3 5’-GGGAGCCTTGAATACACCAAAA-3’ | 500 nM | 0.5 μL |
Reverse primer N3 5’-TGTAGCACGATTGCAGCATTG -3’ | 500 nM | 0.5 μL |
Probe N3 VIC5’-ATCACATTGGCACCCGCAATCCTG-3’BHQ2 | 500 nM | 0.5 μL |
Forward primer UBC 5’-TTGGGTCGCAGTTCTTGTTTG -3’ | 500 nM | 0.5 μL |
Forward primer UBC 5’-TGCCTTGACATTCTCGATGGT-3’ | 500 nM | 0.5 μL |
Forward primer UBC ROX5’-TCGCTGTGATCGTCACTTGACAATG-3’BHQ2 | 500 nM | 0.5 μL |
| ddH2O | 0.5 μL | |
| Total | 5 μL |
Laboratory supplies
1. 8.5 cm Petri dishes (Greiner, Bio-One, catalog number: 633181)
2. 10 cm glass Petri dishes (MiniMed, catalog number: 11000245)
3. Scalpel handle Bayha (GMW, catalog number: 85400)
4. Scalpel blades for Bayha scalpel handles (GMW, catalog number: 85411, FORM 21)
5. TC-treated multiple well plates (6-well plate) (Corning® Costar®, catalog number: CLS3516)
6. 96-well plate polystyrene round-bottom clear wells, sterile (Greiner, catalog number: M2311)
7. Microwell 96-well optical flat-bottom plates (Nunc®, catalog number: P8991)
8. Bottle 500 mL with screw cap GL 45, glass, light (IsoLab, catalog number: 061.01.500)
9. 15 mL conical tubes (SPL Lifesciences, catalog number: 51015)
10. 50 mL conical tubes (SPL Lifesciences, catalog number: 50250)
11. 5 mL tubes Pirouet (SSIbio, catalog number: 1410-S0)
12. 2 mL tubes Pirouet (SSIbio, catalog number: SKU: 1310-S0)
13. 1.5 mL tubes Pirouet (SSIbio, catalog number: 1210-S0)
14. 0.5 mL low-binding tubes Pirouet (SSIbio, catalog number: 1110-10)
15. 1.8 mL cryovials (SPL Lifesciences, catalog number: 43012)
16. 5 mL serological pipettes (Corning® Costar®, catalog number: CLS4487)
17. 10 mL serological pipettes (Greiner Bio-One, catalog number: CLS4488)
18. 25 mL serological pipettes (Corning® Costar®, catalog number: CLS4489)
19. 10 μL pipette tips (TH Geyer, catalog number: 7695881)
20. 20 μL pipette tips (PakGent Bio, catalog number: UFPT-F-20)
21. 200 μL pipette tips (TH Geyer, catalog number: 7695884)
22. 1,250 μL pipette tips (TH Geyer, catalog number: 7695887)
23. CountessTM cell counting chamber slides (Thermo Fisher Scientific, Invitrogen, catalog number: C10283)
24. 40 μm strainers (Corning®, catalog number: CLS431750)
25. Lysing matrix A, bulk, 0.56–0.7-mm garnet flakes (MP Biomedicals, catalog number: 11-654-0423)
Equipment
1. Faster SafeFAST Elite RU 212S Class II biological safety cabinet (SafeFAST, catalog number: 212S)
2. Class II biological safety cabinet BMB-II Laminar-С 1,5 NEOTERIC (Lamsystems, catalog number: 1R-B.001-15)
3. Binder CB60 CO2 incubator (Binder, catalog number: 9040-0090)
4. Binder CB-150 CO2 incubator (Binder, catalog number: 9040-0126/CB150-230V-O-RU)
5. CountessTM II FL automated cell counter (Thermo Fisher Scientific, catalog number: AMQAF1000)
6. Water bath (37–65 °C) (BIOSAN, model: WB-4MS)
7. Freezer (-40 °C) (ALS, model: PLATINUM 500SV)
8. Freezer (-80 °C) (Thermo Scientific, model: FORMA 905)
9. Refrigerator (2–8 °C) (Bonvini, model: 750 BGC)
10. Laboratory centrifuge with different rotors (Hettich, model: ROTINA 380R)
11. Laboratory centrifuge (Eppendorf, model: Centrifuge 5415 R)
12. Laboratory centrifuge (Eppendorf, model: Mini Spin)
13. Inverted microscope (objectives: 4×, 10×, 20×) (Zeiss, model: Primovert)
14. FinnpipetteTM F2 GLP Pipetting kit 2 (Thermo Fisher Scientific, catalog number: 11845850)
15. FACSymphony A5 instrument
16. FastPrep-24 homogenizer (MP Biomedicals, catalog number: 116004500)
17. Multiplex system Luminex 200 (Immucor Transplant Diagnostics, Inc., catalog number: 888310)
18. Automatic magnetic washer (Biotek ELx405, Winooski, VT, USA)
19. Multichannel pipette 5–50 μL Discovery Comfort DV8 (HTL, catalog number: 5122)
20. Multichannel pipette 20–200 μL Discovery Comfort DV8 (HTL, catalog number: 5123)
21. Rubber bands
22. Aluminum foil
23. Absorbent pads
24. Racks
25. Laboratory vortex mixer MICROSPIN (BIOSAN, model: FV-2400)
26. Sonicator ProbeTec ET (BD Ultrasonic Cleaner model: 2510E-DTH)
27. Orbital shaker incubator (BIOSAN, model: ES-20)
28. Thermoshaker for plates (BIOSAN, model: PST-60HL)
Software and datasets
1. Bio-Plex Manager Software (BioRad, Version 4.0)
2. FlowJo v.10
3. FIJI ImageJ (v.1.54)
4. R software (R, Version 4.2.1)
5. The data obtained using this protocol are openly available at https://github.com/GeorgeRusakovich/Cytokine-production-in-ex-vivo-model-of-SARS-CoV-2-lung-infection.
Procedure
文章信息
稿件历史记录
提交日期: Sep 9, 2025
接收日期: Nov 12, 2025
在线发布日期: Nov 28, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Maryukhnich, E. V., Potashnikova, D. M., Vorobyeva, D. A., Rusakovich, G. I., Tvorogova, A. V., Kalinskaya, A. I., Pinegina, N. V., Kovyrshina, A. V., Dolzhikova, I. V., Postnikov, A. B., Rozov, F. N., Sotnikova, T. N., Kanner, D. Y., Logunov, D. Y., Gintsburg, A. L. and Vasilieva, E. J. (2025). An Ex Vivo Lung Histoculture Model for Studying Pulmonary Infection and Immune Response With SARS-CoV-2 as an Example of RNA Virus. Bio-protocol 15(24): e5552. DOI: 10.21769/BioProtoc.5552.
分类
免疫学 > 宿主防御 > 人
细胞生物学 > 细胞分离和培养 > 器官培养
微生物学 > 微生物-宿主相互作用 > 离体模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










