- EN - English
- CN - 中文
Vascularization of Human Pancreatic Islets With Adaptive Endothelial Cells for In Vitro Analysis and In Vivo Transplantation
利用适应性内皮细胞实现人胰岛体血管化,用于体外分析及体内移植
发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5550 浏览次数: 770
评审: Joyce ChiuAnonymous reviewer(s)
Abstract
The pancreatic islet, the only type of tissue that secretes insulin in response to elevated blood glucose, plays a vital role in diabetes development and treatment. While various islet vascularization strategies have been developed, they have been hindered by major limitations such as relying on pre-patterning and the inability to span long distances. Furthermore, few strategies have demonstrated robust enough vascularization in vivo to support therapeutic subcutaneous islet transplantation. Using adaptive endothelial cells (ECs) reprogrammed by transient expression of the ETS Variant Transcription Factor 2 (ETV-2) gene, we have physiologically vascularized human islets within a generic microchamber and have achieved functional engraftment of human islets in the subcutaneous space of mice. Such adaptive ECs, which we term reprogrammed vascular ECs (R-VECs), have been proven to be a suitable tool for both in vitro disease modeling and in vivo transplantation of not only islets but also other organoids.
Key features
• This protocol contains two parts: the in vitro and in vivo parts, both utilizing adaptable endothelial cells to functionally vascularize human islets.
• The in vitro portion of this protocol describes the method to culture human islets in a vascular bed within a large and commercially available microchamber.
• The in vivo portion of this protocol provides a step-by-step procedure to reverse hyperglycemia in streptozotocin-induced diabetic mice.
Keywords: Pancreatic islet (胰岛)Graphical overview
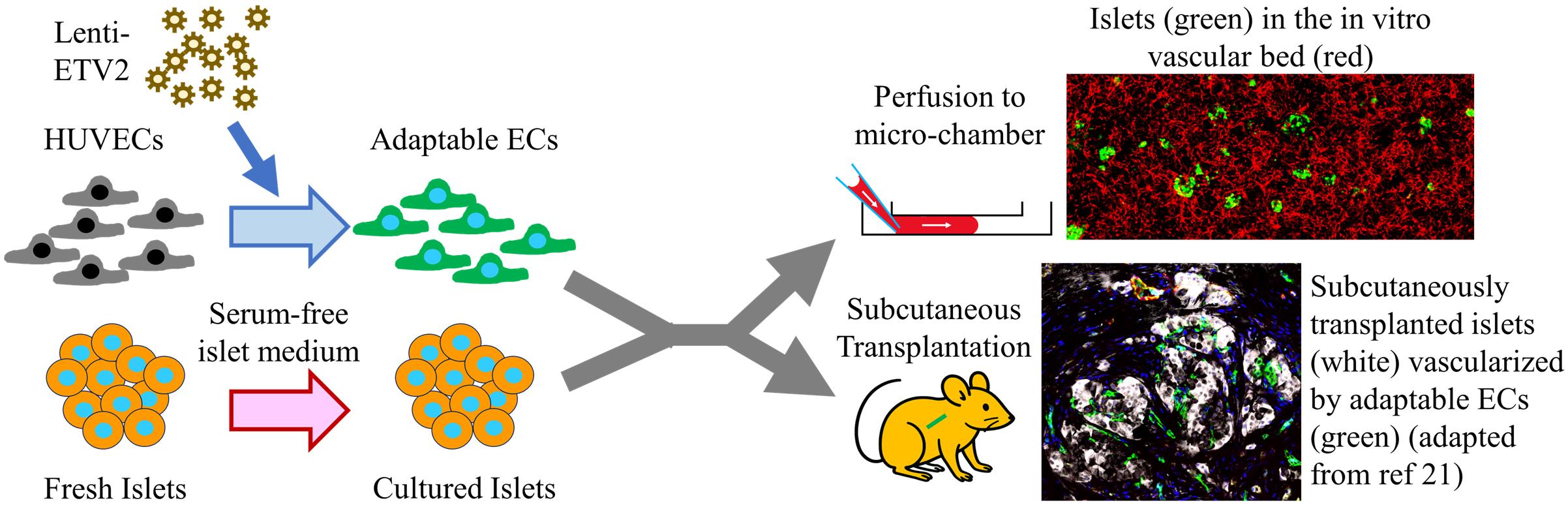
Background
According to the August 2021–August 2023 cycle of the National Health and Nutrition Examination Survey (NHANES) published by the Centers for Disease Control and Prevention, 15.8% of US adults are estimated to have diabetes. Type 1 diabetes (T1D) is caused by the autoimmune destruction of pancreatic islets, the cells that produce insulin in response to elevated glucose levels [1]. In contrast, type 2 diabetes (T2D) generally begins with reduced insulin sensitivity in insulin-responsive tissues, such as the liver, muscles, and adipose tissue. The body often compensates for such insulin resistance by islet proliferation. However, failure in compensatory islet proliferation and islet dysfunction leads to T2D onset [2]. Therefore, strategies that promote islet regeneration or function hold great potential in treating diabetes.
Among the medications used in diabetes treatment, only DPP4 and GLP-1 receptor agonists can potentially improve islet function [3]. No medications have been proven to promote islet regeneration, apart from possibly harmine [4], which is still under clinical trial. One obstacle in the development of islet-targeting drugs is the difficulty in culturing islets in vitro for the long term. Pancreatic islets are spherical clusters of cells with diameters ranging from 50 to 500 μm [5]. A human adult pancreas contains 1–2 million islets, composing approximately 2% of the pancreatic mass. Compared to the acinar and ductal tissues of the pancreas, islets are highly vascular and receive 20% of the blood supply to the pancreas [6]. The most commonly used protocol for isolating human islets uses the Ricordi automated method [7], which utilizes physical forces and enzymatic reactions to separate islets from exocrine tissues and purifies islets by gradient centrifugation. To then culture the isolated islets, the two most commonly used media are RPMI 1640 supplemented with 10% serum or serum-free Connaught’s Medical Research Lab (CMRL) medium, neither of which can support normal islet function for more than a week [8]. Commercial media with undisclosed recipes exist, such as the PIM(S)® from Prodo Lab, which may permit long-term islet culture for up to 21 days, as reported by the company’s documents. It therefore remains a challenge to sustain islet function long-term in vitro.
Part of the difficulty in long-term culturing of islets is due to the fact that islet isolation protocols disrupt the islets’ rich vascular connections. Most endothelial cells within the islet are believed to deteriorate soon after isolation, leaving cells within the center of the avascular islet with limited nutrients and oxygen [9]. Various strategies to vascularize in vitro cultured islets include prefabricated chips [10] or bioprinting [11]. These strategies require highly specialized techniques and do not achieve an intra-islet vascular density comparable to that of native islet capillaries. In this protocol, we convert generic endothelial cells (ECs) into adaptable ECs by overexpression of the endothelial pioneer transcription factor ETV-2. Adaptable ECs functionally vascularize islets in vitro and sustain islet function for more than 20 weeks. This approach provides an ideal platform for screening therapeutics to promote islet functional improvement or regeneration.
Beyond drugs to augment islet functional mass, islet β-cell replacement therapy is considered another promising treatment for diabetes, which provides finely tuned insulin secretion at physiological levels by transplanting insulin-secreting islet cells derived from either human deceased donors or from stem cells [12]. Clinically, islet transplantation is used to treat T1D patients with severe hypoglycemic complications. There are several obstacles preventing cellular therapy from expanding to a wider T1D population or even to T2D patients, including the use of immunosuppressants, scarcity of donor cells, and high costs. Furthermore, clinical transplantation protocols involve the infusion of either deceased donor-derived or stem cell–derived islets into the portal vein of the liver [13]. Portal infusion of transplanted cells carries with it the small but very serious risk of thrombosis, also limiting the infusion of anything larger than naked islets. Therefore, attempts to encapsulate islets for immune shielding have involved alternative transplantation sites such as the subcutaneous space [14] and the omentum [15]. Among the various alternative transplantation sites, the subcutaneous space is the most promising due to its large size, ease of access and monitoring, and ability to retrieve transplanted cells. Compared to most other sites, such as the kidney capsule, omentum, and anterior chamber of the eye, the subcutaneous space is enormous, making it ideal to construct sophisticated structures not only to protect grafts from immune attack but to establish a tissue-specific vascular niche to sustain islet functional mass. Transplanting islets in the subcutaneous space, however, has long been hampered by the difficulty in establishing a sufficient blood supply to the grafts [16,17]. In this protocol, we present a method of co-transplanting islets with ETV-2-expressing adaptable ECs to achieve functional engraftment of human islets within the subcutaneous space.
Materials and reagents
Biological materials
1. Human umbilical vein endothelial cells (HUVECs)
Note: HUVECs were generated in-house following a published protocol [18]. Briefly, endothelial cells were digested from freshly acquired umbilical cord veins through collagenase digestion, followed by culturing in human EC medium in gelatin-coated 10-cm tissue culture dishes. The 10-cm tissue culture dish was coated by incubating with 4 mL of 0.1% gelatin for at least 30 min at 37 °C.
2. Lentivirus carrying hPGK-driven ETV2 (Lenti-ETV2); constructed and ordered from VectorBuilder (the construction map of the lentivirus vector plasmid is shown in Figure 1)
3. Adeno-associated virus carrying hPGK-driven EGFP (AAV-EGFP); ordered from VectorBuilder
4. Human islets, obtained from the following three sources: the Integrated Islet Distribution Program (IIDP), Columbia Center for Translational Immunology (CCTI), or Prodo Laboratories Inc.
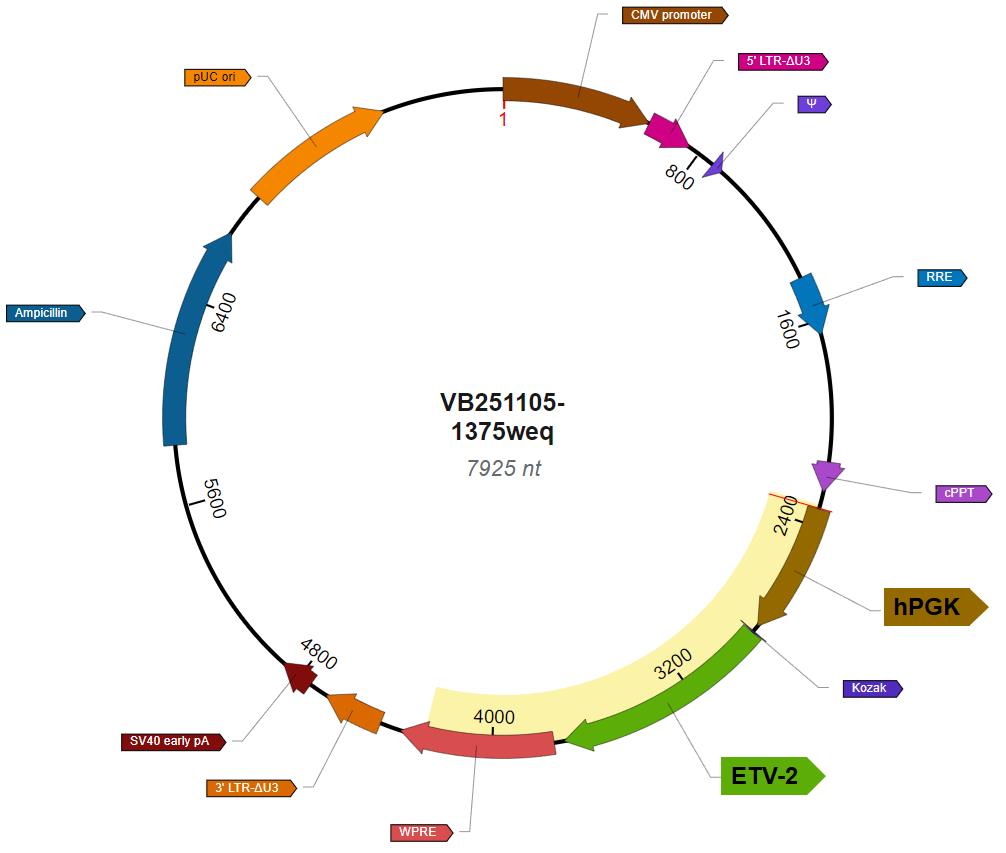
Figure 1. Vector map of Lenti-ETV2
Experimental animals
All mice used in this research were either purchased from Charles River Laboratories or bred at Weill Cornell Animal Facility. All animal work was approved by the Weill Cornell IACUC committee, with the protocol numbers 2009-0611 and 2023-0026.
Reagents
1. Medium 199, Earle's salts (Thermo Fisher Scientific, GibcoTM, catalog number: 11150067)
2. Penicillin-streptomycin-neomycin (PSN) antibiotic mixture (Thermo Fisher Scientific, GibcoTM, catalog number: 15640055)
3. GlutaMAXTM supplement (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061)
4. Chemically defined lipid concentrate (Thermo Fisher Scientific, GibcoTM, catalog number: 11905031)
5. HEPES (1 M) (Thermo Fisher Scientific, GibcoTM, catalog number: 15630080)
6. Heparin sodium salt from porcine intestinal mucosa (Millipore Sigma, catalog number: H3149-10KU)
7. N-Acetyl-cysteine (Millipore Sigma, catalog number: A9165-5G)
8. Human FGF-basic recombinant protein (Thermo Fisher Scientific, PeproTech®, catalog number: 100-18B)
9. Human IGF-I recombinant protein (Thermo Fisher Scientific, PeproTech®, catalog number: 100-11)
10. Human EGF recombinant protein (Thermo Fisher Scientific, PeproTech®, catalog number: AF-11-15)
11. TheraPEAK® X-VIVO® 10 (Lonza, catalog number: BEBP04-743Q)
12. Fibrinogen from bovine plasma (Millipore Sigma, catalog number: 341573)
13. Thrombin from bovine plasma (Millipore Sigma, catalog number: T4648)
14. StemSpanTM SFEM (STEMCELL Technology, catalog number: 09650)
15. KnockOutTM serum replacement (Thermo Fisher Scientific, GibcoTM, catalog number: 10828028)
16. RPMI 1640 medium (Thermo Fisher Scientific, GibcoTM, catalog number: 11875093)
17. AlbuMAXTM II lipid-rich BSA (Thermo Fisher Scientific, GibcoTM, catalog number: 11021029)
18. Transferrin human (Millipore Sigma, catalog number: T8158)
19. Human recombinant prolactin (STEMCELL Technology, catalog number: 78098.1)
20. Ethanolamine (Millipore Sigma, catalog number: 15014)
21. 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (Millipore Sigma, catalog number: 42490)
22. Sodium selenite (Millipore Sigma, catalog number: 71950)
23. HEPES, suitable for cell culture (Millipore Sigma, catalog number: H4034)
24. Bovine serum albumin (BSA), suitable for cell culture (Millipore Sigma, catalog number: A1470)
25. Fluo-4 DirectTM Calcium Assay kit (Thermo Fisher, InvitrogenTM, catalog number: F10471)
26. Streptozotocin (STZ) (Millipore Sigma, catalog number: S0130)
27. Accutase® (Thermo Fisher Scientific, catalog number: 00-4555-56)
28. Isoflurane (Covetrus, catalog number: 11695-6777-1)
29. Matrigel® growth factor reduced (GFR) basement membrane matrix (Corning, catalog number: 356231)
30. 10× DMEM (low glucose) (Millipore Sigma, catalog number: D2429)
31. Bovine type 1 collagen (Advanced Biomatrix, catalog number: 5225)
32. Insulin Chemiluminescence ELISA Jumbo Pack (Alpco, catalog number: 80-INSHU-CH10)
33. Gelatin (Carolina, catalog number: 864660)
34. Matrigel (Corning, catalog number: 356237)
Solutions
1. Human EC medium (see Recipes)
2. Tube medium (see Recipes)
3. Serum-free islet (SFI) medium (see Recipes)
4. Krebs-Ringer bicarbonate solution, HEPES-buffered (KRBH buffer) (see Recipes)
5. Cryopreservation solution (see Recipes)
Recipes
1. Human EC medium
| Component | Volume or final concentration |
|---|---|
| Medium 199, Earle's salts | 500 mL |
| Penicillin-streptomycin-neomycin antibiotic mixture | 6.25 mL |
| GlutaMAXTM supplement | 6.25 mL |
| HEPES (1 M) | 9.375 mL |
| Chemically defined lipid concentrate | 6.25 mg/mL |
| Heparin sodium salt from porcine intestinal mucosa | 0.1 mg/mL |
| Fetal bovine serum | 125 mL |
| N-Acetyl-L-cysteine | 1 mM |
| Human FGF-basic recombinant protein | 10 ng/mL |
| Human IGF-I recombinant protein | 10 ng/mL |
| Human EGF recombinant protein | 10 ng/mL |
| Total | ~650 mL |
2. Tube medium
| Components | Proportion | Volume |
|---|---|---|
| StemSpanTM SFEM | 90% | 45 mL |
| KnockOutTM serum replacement | 10% | 5 mL |
| Total | 100% | 50 mL |
3. Serum-free islet (SFI) medium
| Components | Volume or final concentration |
|---|---|
| RPMI 1640 medium | 500 mL |
| AlbuMAXTM II lipid-rich BSA | 0.20% |
| Transferrin human | 10 μg/mL |
| Human prolactin recombinant protein | 20 ng/mL |
| Human IGF-I recombinant protein | 10 ng/mL |
| Human FGF-basic recombinant protein | 10 ng/mL |
| Ethanolamine | 50 μM |
| 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine | 50 μM |
| Sodium selenite | 6.7 μg/mL |
| Heparin sodium salt from porcine intestinal mucosa | 100 ng/mL |
| Total | ~500 mL |
4. Krebs-Ringer bicarbonate solution, HEPES-buffered (KRBH buffer)
| Component | Final concentration |
|---|---|
| NaCl | 128.8 mM |
| KCl | 4.8 mM |
| KH2PO4 | 1.2 mM |
| MgSO4·7H2O | 1.2 mM |
| CaCl2 | 2.5 mM |
| NaHCO3 | 5 mM |
| HEPES | 10 mM |
| D-glucose | 2 mM |
| Bovine serum albumin | 0.1% |
5. Cryopreservation solution
| Components | Proportion | Volume |
|---|---|---|
| Human EC medium | 40% | 20 mL |
| Fetal bovine serum | 50% | 25 mL |
| DMSO | 10% | 5 mL |
| Total | 100% | 50 mL |
Laboratory supplies
1. 1 mL syringe (BD, catalog number: 309659)
2. 5 mL syringe (BD, catalog number: 309647)
3. μ-slide VI 0.4 (Ibidi, catalog number: 80606)
Equipment
1. Cell incubator (e.g., Panasonic, model: MCO-19M-PA or any cell incubator that is capable of maintaining the temperature at 37 °C and the CO2 level at 5%)
2. Biosafety cabinet (e.g., Baker, model: SterilGARD SG6C3A-HE or any certified class 2 biosafety cabinet designed for cell culture)
3. Heated veterinary operating table (e.g., PECO Services, model: V500DVStat or any heated pad that can sustain a temperature of 37 °C)
4. Fluorescence microscope (e.g., Confocol.nl NL5+ or any confocal or fluorescence microscope with the capability of taking time-lapse images at one acquisition per second and with an environmental chamber to maintain sample temperature at 37 °C)
Procedure
文章信息
稿件历史记录
提交日期: Sep 12, 2025
接收日期: Nov 13, 2025
在线发布日期: Dec 2, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Li, G., Craig-Schapiro, R., Uddin, A. and Rafii, S. (2025). Vascularization of Human Pancreatic Islets With Adaptive Endothelial Cells for In Vitro Analysis and In Vivo Transplantation. Bio-protocol 15(24): e5550. DOI: 10.21769/BioProtoc.5550.
- Li, G., Craig-Schapiro, R., Redmond, D., Chen, K., Lin, Y., Geng, F., Gao, M., Rabbany, S. Y., Suresh, G., Pearson, B., et al. (2025). Vascularization of human islets by adaptable endothelium for durable and functional subcutaneous engraftment. Sci Adv. 11(5): eadq5302. https://doi.org/10.1126/sciadv.adq5302
分类
细胞生物学 > 细胞工程 > 组织工程
细胞生物学 > 细胞移植 > 异种移植
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












