- EN - English
- CN - 中文
In Situ Crosslinking of Bioorthogonal Nanoparticles to Restore Clot Stability in Coagulopathic Blood
原位交联生物正交纳米颗粒以恢复凝血障碍血液中的血栓稳定性
(§Technical contact: celestine.hong@ubc.ca) 发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5548 浏览次数: 591
评审: Joyce ChiuAnonymous reviewer(s)
Abstract
Intravenous hemostats have shown significant promise in prolonging survival for severe noncompressible and internal injuries in preclinical animal models. Existing approaches include the use of liposomes with or without procoagulant enzymes, as well as polymer nanoparticles or soluble biopolymers. While these methods predominantly target or mimic tissue components that are present during coagulation, such as activated platelets and collagen, they may not account for the loss of fibrinogen, which is not only key to clot formation but also the first protein to fall below critical levels in dilutional coagulopathy. This protocol describes the synthesis and in vitro or ex vivo characterization of a crosslinkable nanoparticle system that seeks to address dilutional coagulopathy by leveraging the critical gelation concentration and bioorthogonal click chemistry. The system was shown to only gel at high nanoparticle and crosslinker concentrations, increase the rate of platelet recruitment, and decrease the rate of clot degradation in a low-fibrinogen environment, providing a platform for treating severe hemorrhage in a coagulopathic environment. Ultimately, the contents of this protocol may assist researchers in the in vitro characterization and screening of other crosslinkable nanoparticle systems or hemostats, with potential expansions to other categories of coagulation dysfunction, such as embolism treatment.
Key features
• A protocol for the synthesis of nanoparticles with activated-platelet-binding moieties to mimic fibrin.
• In vitro and ex vivo assays assessing complement activation, accumulated platelet recruitment, platelet recruitment under hemodilution, coagulation potential, and clot lysis.
• The inclusion of hemodiluted and plasminolytic conditions creates a more physiologically relevant environment for screening of hemostatic agents.
• The use of a two-component system helps reduce complement activation in intravenous therapies.
Keywords: Hemostasis (止血)Graphical overview
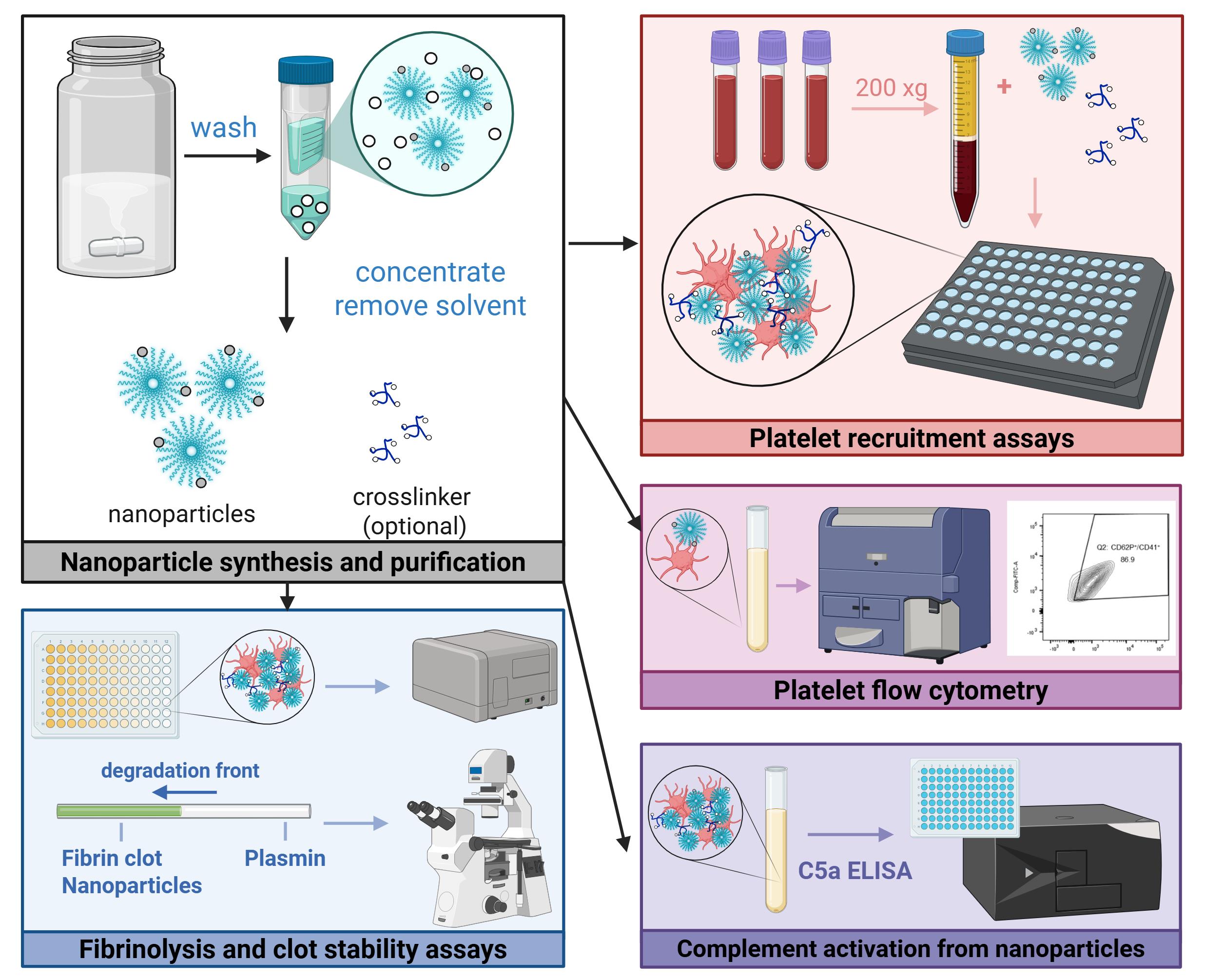
Graphical abstract of experimental protocols
Background
Achieving hemostasis for unknown or inaccessible locations presents a challenge for both embolic and hemorrhagic circumstances, often necessitating surgery or the systemic delivery of therapeutics to stabilize the patient [1–3]. In particular, hemostasis for severe hemorrhage poses additional hurdles to treatment, such as dilutional coagulopathy and potential off-target effects of procoagulant therapeutics. Researchers in the field have sought to address the latter issue by functionalizing liposomes [4,5], nanoparticles, or soluble polymers with targeting moieties that interact with activated components of the coagulation system. However, these solutions do not directly address fibrinogen loss, which is critical to achieving hemostasis, is associated with significantly lower survival rates, and can be further exacerbated by fluid resuscitation measures [6,7]. Moreover, the assays used to screen these materials have been largely limited to flow-based assays, platelet aggregation assays, or rotational thromboelastometry (ROTEM), which may not allow for high-throughput screening of various therapeutics or assess important metrics such as fibrinolytic activity or complement activation.
To this end, we developed a two-component system of dibenzocyclooctyne-functionalized multiarm polymers and nanoparticles with azide and activated-platelet-binding moieties, which leverages the increased accumulation of nanoparticles at the injury [8] to achieve crosslinking above a critical concentration, thereby mimicking the presence of fibrin at the wound site. This platform was demonstrated to result in significantly greater platelet recruitment in both normal and hemodiluted systems, increased resistance to fibrinolysis, and prolonged survival in a mouse liver resection model when compared to targeted nanoparticle-only controls.
The following sections describe in detail the in vitro and ex vivo assays used to characterize hemostats in three key areas: platelet recruitment (hemodiluted and non-hemodiluted conditions), complement activation, and clot strength. While previously described in literature, the nanoparticle synthesis and purification procedure is also included to ensure reproducibility. Ultimately, this protocol may be useful for the characterization of other crosslinkable nanoparticle systems, as well as the assessment of platelet or clot interactions in other modes of coagulation dysfunction.
Materials and reagents
Biological materials
1. Fresh whole blood, room temperature, NaCitrate anticoagulant (Research Blood Components)
2. Platelet-rich plasma (PRP), isolated from the above by centrifuging at 200× g for 20 min with no brake; use within 24 h
Reagents
The reagents below have been organized by experiment. As such, you may see duplicate entries of commonly used items, such as buffers, salts, and platelet agonists. You should have all the items under general/buffers readily available before the experiment.
General/buffers
1. Deionized water
2. Sodium chloride (VWR, catalog number: BDH9286-500G)
3. Calcium chloride (Millipore Sigma, catalog number: 1023780500)
4. HEPES buffer (Millipore Sigma, catalog number: H3375)
5. α-D-Glucose (Millipore Sigma, catalog number: 158968-25G)
Nanoparticle synthesis and purification
1. GRGDS, azide, DBCO, Cy7, or methoxy-functionalized poly(ethylene glycol)-b-poly(D,L-lactide-co-glycolide) (PEG-PLGA), 25–35 kDa, lactide:glycolide ratio 60:40–55:45 (Custom, synthesized as previously described [8,9], frozen at -20 °C)
2. Resomer 503H PLGA (Millipore Sigma, catalog number: 719870-5G, stored at 4 °C)
3. N, N-Dimethylformamide (DMF), anhydrous, 99.8% (Millipore Sigma, catalog number: 227056-100ML)
4. Tetrahydrofuran, 250 ppm BHT inhibitor (THF) anhydrous, ≥99.9% (Millipore Sigma, catalog number: 186562-100ML)
5. 4-arm-PEG-azide, MW 20k (CreativePEGworks, catalog number: PSB-4905-1g, frozen at -20 °C)
6. 4-arm-PEG, MW 20k (Millipore Sigma, JKP2005R-1G, frozen at -20 °C)
7. Deionized water
Platelet recruitment assays (hemodiluted and normal conditions)
1. Pierce RIPA buffer (Thermo Fisher, catalog number: 89901, stored at 4 °C)
2. Invitrogen CyQuant LDH assay (Thermo Fisher, catalog number: C20301, stored at 4 °C)
3. Adenosine 5’-diphosphate sodium salt (Millipore Sigma, catalog number: A2754-1G, frozen at -20 °C)
4. Sodium chloride (VWR, catalog number: BDH9286-500G)
5. Deionized water
Fibrin crosslinking in hemodiluted conditions
1. Calcium chloride (Millipore Sigma, catalog number: 1023780500)
2. Sodium chloride (VWR, catalog number: BDH9286-500G)
3. Deionized water
Platelet flow cytometry
1. Bovine serum albumin (BSA) (Millipore Sigma, catalog number: A3608)
2. Thrombin from human plasma (Millipore Sigma, catalog number: T6884-250UN)
3. H-Gly-Pro-Arg-Pro-NH2 (GPRP) acetate salt (Bachem, catalog number: 4025347.0025)
4. Calcium chloride (Millipore Sigma, catalog number: 1023780500)
5. Sodium chloride (VWR, catalog number: BDH9286-500G)
6. AF488 anti-human CD41 antibody (BioLegend, catalog number: 303724)
7. Brilliant Violet 421TM anti-human CD62P (P-Selectin) antibody (BioLegend, catalog number: 304925)
8. Pierce 16% formaldehyde (w/v), methanol-free (Thermo Fisher, catalog number: 28906)
Evaluating complement activation from nanoparticles
1. Invitrogen Complement C5a Human ELISA kit (Thermo Fisher, catalog number: BMS2088)
2. Zymosan A from Saccharomyces cerevisiae (Millipore Sigma, catalog number: Z4250-250MG)
3. Sodium chloride (VWR, catalog number: BDH9286-500G)
4. Deionized water
Fibrin clot lysis capillary assay
1. Fibrinogen, plasminogen-depleted, human plasma (Millipore Sigma, catalog number: 341578)
2. AF488 NHS ester (Lumiprobe, catalog number: 21820)
3. Plasmin (Millipore Sigma, catalog number: P1867-500UG)
4. Thrombin from human plasma (Millipore Sigma, catalog number: T6884-250UN)
5. Sodium chloride (VWR, catalog number: BDH9286-500G)
6. Deionized water
Solutions
1. Isotonic saline (see Recipes)
2. Isotonic glucose (see Recipes)
3. Adenosine diphosphate (ADP) stock solution (see Recipes)
4. Plasmin stock (see Recipes)
5. Calcium chloride (see Recipes)
6. Thrombin stock (see Recipes)
7. GPRP (see Recipes)
8. FACS buffer (see Recipes)
9. Diluted activated PRP (see Recipes)
Recipes
Note: All aliquoted solutions should be thawed, used, and discarded. No solutions should undergo repeated freeze-thaw cycles.
1. Isotonic saline
Prepare isotonic saline by diluting sodium chloride in deionized water to 0.9% w/v.
2. Isotonic glucose
Prepare isotonic glucose by diluting glucose in deionized water to 5% w/v. You may also prepare a 10× solution by preparing a solution of 50% w/v glucose (which may need to be dissolved in a warm water bath at 40 °C and filter-sterilized).
3. Adenosine diphosphate (ADP) stock solution
Prepare the ADP solution at a concentration of 0.1 mM in isotonic saline. Divide the solution into 100 μL aliquots and store at -20 °C.
4. Plasmin stock
Prepare the plasmin solution at a concentration of 10 μg/mL in isotonic saline. Divide the solution into 1 mL aliquots and store at -20 °C.
5. Calcium chloride stock
Prepare the calcium chloride solution at a concentration of 100 mM in isotonic saline.
6. Thrombin stock
Prepare the thrombin stock at a concentration of 100 U/mL in isotonic saline. Divide the solution into 100 μL aliquots and store at -20 °C.
7. GPRP stock
Prepare the GPRP solution at a concentration of 15 mM in isotonic saline. Divide the solution into 150 μL aliquots and store at -20 °C.
8. FACS buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride | 0.9% (w/v) | 450 mg |
| BSA | 5% (w/v) | 5 g |
| Deionized water | Begin with 90 mL and fill | |
| Total | n/a | 100 mL |
9. Diluted activated PRP
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Calcium chloride (100 mM) | 3 mM | 15 μL |
| Thrombin (100 U/mL) | 20 U/mL | 100 μL |
| GPRP (15 mM) | 4.5 mM | 150 μL |
| PRP | n/a | 150 μL |
| FACS buffer | n/a | 85 μL |
| Total | n/a | 500 μL |
Laboratory supplies
1. 20 mL scintillation vials (VWR, catalog number: 66022-106)
2. 7 mL scintillation vials (VWR, catalog number: 66022-300)
3. Amicon Ultra centrifugal filters, 3kDa MWCO (Millipore Sigma, catalog number: UFC900308, 15 mL)
4. Eppendorf tubes (Millipore Sigma, catalog number: EP022364111-1EA)
5. FalconTM 50 mL high clarity conical centrifuge tubes (Fisher Scientific, catalog number: 14-959-49A)
6. Greiner CELLSTAR black cell culture microplate, F-bottom, 96 well (Fisher Scientific, catalog number: 07000135)
7. Corning black microplate, clear-bottom, 384 well (Millipore Sigma, catalog number: CLS3764-20EA)
8. Costar non-treated clear polystyrene plates, 96 well (Corning, catalog number: 3370)
9. FalconTM round-bottom polystyrene test tubes with cell strainer snap cap, 5 mL (Fisher Scientific, catalog number: 08-771-23)
10. Square glass tubing (VitroTubesTM) (VitroCom, catalog number: 8100)
11. VWR® shell vial (Avantor, catalog number: 66015-702A)
12. HydroLogixTM ART pipette tips, gel loading (Thermo Scientific, Avantor, catalog number: 53225-648)
13. Dow CorningTM high vacuum grease (Fisher Scientific, catalog number: AA44224KT)
Equipment
1. Plate reader (Tecan, model: Infinite M200)
2. Plate reader (Tecan, model: M Nano)
3. Fluorescent microscope (Olympus, model: IX83; camera model: DP30BW)
4. Bath sonicator (Branson, model: 2510EDTH)
5. Flow cytometer (BD Biosciences, model: LSR II)
Software and datasets
1. Prism (GraphPad, Version 5, lifetime license)
2. FlowJo (Version 10)
3. BioRender (https://www.biorender.com/)
Procedure
文章信息
稿件历史记录
提交日期: Sep 30, 2025
接收日期: Nov 12, 2025
在线发布日期: Nov 26, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Hong, C., He, Y., Belcher, A. M., Olsen, B. D. and Hammond, P. T. (2025). In Situ Crosslinking of Bioorthogonal Nanoparticles to Restore Clot Stability in Coagulopathic Blood. Bio-protocol 15(24): e5548. DOI: 10.21769/BioProtoc.5548.
分类
生物工程 > 生物医学工程 > 药物递送
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












