- EN - English
- CN - 中文
Plasmid DNA Purification Using Filterprep With an Optional Endotoxin Removal Step
基于 Filterprep 的质粒 DNA 纯化方法及可选内毒素去除步骤
发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5547 浏览次数: 1106
评审: Hemant Kumar PrajapatiAnonymous reviewer(s)

相关实验方案

深海无脊椎动物组织保存的比较方案:DNA/RNA Shield 与液氮在高质量核酸双重提取中的应用对比
Ana S. Gomes [...] Olivier Laroche
2025年11月20日 1335 阅读
Abstract
This protocol presents a modified version of the Filterprep method originally reported in New Biotechnology, adding an optional step to reduce endotoxin levels. Filterprep is a simple, rapid, and cost-effective approach to plasmid DNA purification that couples ethanol precipitation with a single spin-column filtration step, eliminating chaotropic salts and silica binding. The formulations and parameters are fully transparent and do not rely on proprietary buffers, using only standard laboratory reagents and widely available miniprep columns. Under matched conditions, the method recovers high-purity plasmid DNA with yields up to fivefold higher than those obtained with representative commercial midiprep kits. The workflow is readily adoptable in most molecular biology laboratories and, under routine conditions, can be completed in approximately 40 min. The resulting DNA is suitable for molecular cloning, PCR, sequencing, and other downstream biochemical applications. Endotoxin is a lipopolysaccharide (LPS) found in the outer membrane of Gram-negative bacteria and may carry over during plasmid preparation. For experiments requiring lower endotoxin input, an optional modification resuspends the DNA pellet in a Triton X-114 wash buffer before column loading to decrease lipopolysaccharide carryover. The method is modular and extensible, allowing adjustment of precipitation and wash conditions, variation in the number of washes, selection of alternative column formats, and integration of endotoxin-reduction modules without altering the core principle. These features facilitate troubleshooting and quality control, enable scaling from routine batches to larger culture volumes and higher throughput, and allow seamless integration with existing workflows.
Key features
• Modular, chaotrope-free workflow [1] combining ethanol precipitation and single-column cleanup; transparent chemistry allows RNase, endotoxin reduction, or extra washes without changing the core principle.
• Uses only standard reagents, a microcentrifuge, and common miniprep columns, with no proprietary kits, vacuum manifolds, or specialized equipment, enabling broad adoption across laboratories.
• Extensible across scales from small miniprep volumes to larger cultures while remaining compatible with cloning, PCR, sequencing, and transfection-grade applications.
• Optional low-endotoxin modification resuspends the DNA pellet in Triton X-114 wash buffer before column filtration to reduce lipopolysaccharide carryover.
Keywords: Plasmid DNA purification (质粒 DNA 纯化)Graphical overview
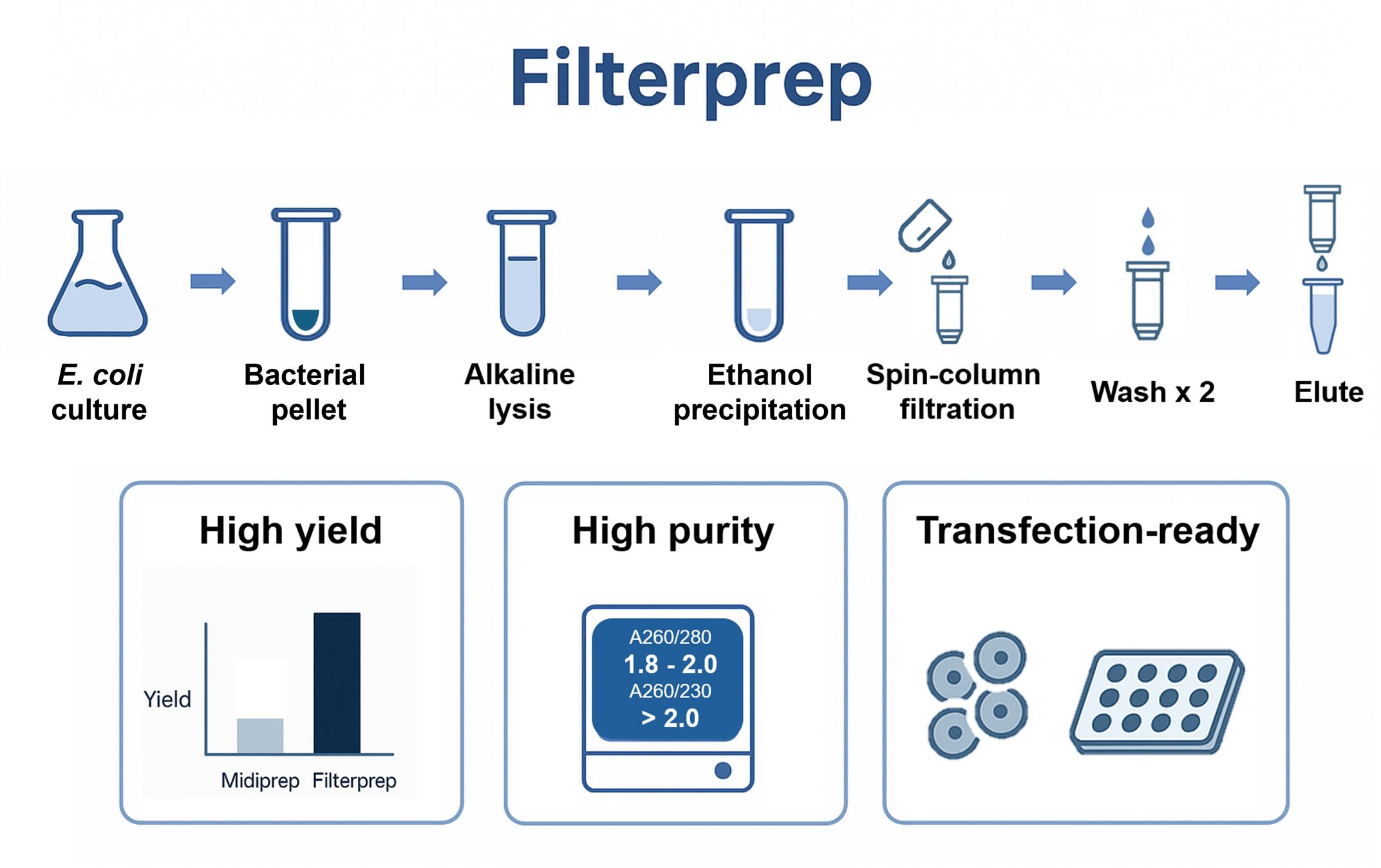
Filterprep hybrid workflow for plasmid purification. Chaotrope-free workflow using ethanol precipitation for capture and a spin column for transfer and washing, delivering high yield and purity with broad downstream compatibility.
Background
Plasmid DNA purification is foundational to molecular cloning, genome engineering, and functional genomics [2–5]. Standard workflows couple alkaline lysis with silica spin columns or magnetic beads. While convenient, these approaches often rely on proprietary formulations and chaotropic salts, which increase cost and limit flexibility [6]. Traditional non-column methods, such as phenol/chloroform extraction with ethanol precipitation or CsCl/ethidium bromide ultracentrifugation, are feasible but require organic solvents and waste handling, involve lengthy and operator-sensitive procedures, and show greater batch-to-batch variability, which reduces suitability for high-throughput, routine use [7,8].
Filterprep combines ethanol precipitation with a single spin column used as a physical filter rather than a silica-binding matrix. The chemistry is transparent and chaotrope-free, relies only on routine reagents and widely available miniprep columns, and is straightforward to implement across laboratories [1]. Under matched conditions, it yields high-purity plasmid DNA and can outperform representative commercial midiprep kits while remaining compatible with molecular cloning, PCR, sequencing, and transfection [9–11].
Endotoxin is a lipopolysaccharide (LPS) derived from the outer membrane of Gram-negative bacteria and may be carried over into plasmid DNA preparations. Although such background levels are generally acceptable for routine applications, some experimental contexts, such as the transfection of sensitive cell lines or preclinical testing, may require reduced endotoxin content. To address this, an optional modification resuspends the DNA pellet in a Triton X-114 wash buffer prior to column loading, thereby decreasing lipopolysaccharide carryover. Prior studies have shown that incorporating a nonionic surfactant such as Triton X-114 directly into the wash buffer can reduce endotoxin carryover without compromising recovery, consistent with the micellar sequestration of LPS [12–14]. This step fits within the existing timeline and requires no additional equipment, making it practical when lower endotoxin input is desired.
Compared with silica-based kits, the protocol reduces dependence on chaotropic salts and proprietary buffers, lowers per-sample cost, and facilitates troubleshooting and quality control, while also avoiding the organic solvents and ultracentrifugation required by traditional non-column methods. These properties shorten hands-on time, reduce batch-to-batch variability, and improve process predictability. Overall, Filterprep supports routine plasmid preparation and method development. Because of its modular design and parameter transparency, it has the potential to extend from routine scale to larger culture volumes and higher throughput by proportionally increasing precipitation and wash volumes, running multiple columns in parallel, or using higher-capacity formats. It can also serve as an upstream pretreatment before downstream polishing and stringent endotoxin-control modules, providing a pathway toward pilot-scale development and industrial application.
Materials and reagents
Biological materials
1. Fast-TransTM competent E. coli DH5α cells (Protech, catalog number: PT-FTDH5)
2. pEGFP-C1 plasmid (Clontech, catalog number: 6084-1)
Reagents
1. LB broth (Miller) (BioShop, catalog number: LBL407)
2. Trizma® base (Sigma, catalog number: T1503)
3. EDTA (BioShop, catalog number: EDT001)
4. Thymolphthalein (Sigma, catalog number: 114553)
5. RNase A (100 mg/mL) (QIAGEN, catalog number: 19101)
6. Hydrochloric acid (HCl) (Merck, catalog number: 1.13134)
7. Potassium acetate (KOAc) (BioShop, catalog number: POA301)
8. Glacial acetic acid (Sigma, catalog number: A6283)
9. Sodium dodecyl sulfate (SDS) (Sigma, catalog number: 75746)
10. Sodium hydroxide (NaOH) (Sigma, catalog number: 221465)
11. 95% ethanol (Merck, catalog number: 1.00983)
12. 100% ethanol (Sigma, catalog number: 459844)
13. Triton X-114 (Sigma, catalog number: 93422)
Solutions
1. 1 M thymolphthalein (see Recipes)
2. Solution 1 (see Recipes)
3. Solution 2 (see Recipes)
4. Solution 3 (see Recipes)
5. Wash buffer (see Recipes)
6. Endotoxin removal buffer (ETR Buffer) (see Recipes)
Recipes
1. 1 M thymolphthalein
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Thymolphthalein | 1 M | 4.3 g |
| 100% ethanol | to a final volume of 10 mL |
2. Solution 1
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-HCl, pH 8.0 | 50 mM | 25 mL |
| 0.5 M EDTA, pH 8.0 | 10 mM | 10 mL |
| 1 M thymolphthalein (optional) | 1 mM | 0.5 mL |
| ddH2O | to a final volume of 500 mL |
3. Solution 2
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaOH | 0.2 N | 4 g |
| SDS | 1% | 5 g |
| ddH2O | to a final volume of 500 mL |
3. Solution 3
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| KOAc | 3 M | 147.2 g |
| ddH2O | 400 mL | |
| Glacial acetic acid | Titrate to pH 5.5 | |
| ddH2O | to a final volume of 500 mL |
4. Wash buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-HCl, pH 7.5 | 10 mM | 5 mL |
| 100% ethanol | 80% | 400 mL |
| ddH2O | n/a | 95 mL |
| Total | n/a | 500 mL |
5. Endotoxin removal buffer (ETR buffer)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Wash buffer | n/a | 99.8 mL |
| Triton X-114 | 0.2% | 0.2 mL |
Laboratory supplies
1. 14 mL culture tube (Greiner Bio-One, catalog number: 187261)
2. 250 mL baffled flask (DURAN, catalog number: 212833655)
3. KimwipesTM (Kimberly-Clark, catalog number: 34155)
4. 20 mL syringe (TERUMO, catalog number: SS-20L)
5. 50 mL conical centrifuge tube (Greiner Bio-One, catalog number: 227261)
6. PrestoTM Mini Plasmid kit (Geneaid, catalog number: PDH300) (only spin columns were used; buffers were not included in this protocol)
Equipment
1. PH meter (METTLER TOLEDO, model: F20)
2. Centrifuge (Eppendorf, model: 5427R; rotor FA-45-30-11)
3. Centrifuge (KUBOTA, model: 5922; rotor RA-410M3)
4. NanoDrop (Thermo Fisher Scientific, model: NanoDrop One)
Procedure
文章信息
稿件历史记录
提交日期: Oct 9, 2025
接收日期: Nov 18, 2025
在线发布日期: Nov 26, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Lin, Y., Shih, Y. and Chang, C. (2025). Plasmid DNA Purification Using Filterprep With an Optional Endotoxin Removal Step. Bio-protocol 15(24): e5547. DOI: 10.21769/BioProtoc.5547.
分类
分子生物学 > DNA > DNA 提取
微生物学 > 微生物遗传学 > 质粒
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










