- EN - English
- CN - 中文
Stimulation-Guided AAV Delivery and Longitudinal Assessment of Optogenetic Expression in Rat Motor Nerves
刺激引导的 AAV 递送及大鼠运动神经中光遗传表达的纵向评估
发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5545 浏览次数: 606
评审: Anonymous reviewer(s)
Abstract
Optogenetic stimulation of peripheral motor nerves is a promising technique for modulating neural activity via illumination of light-sensitive ion channels known as opsins. Stimulating muscle activity through this method offers many advantages, such as a physiological recruitment order of motor units, reduced fatigue, and target-specific stimulation, which make it a favorable option for use in many neuroscience and motor rehabilitation applications. To enable such optical stimulation, opsin expression in peripheral nerves can be achieved either with transgenic animal models or through injection of viral vectors. In this protocol, we describe a method for driving peripheral nerve opsin expression via intramuscular adeno-associated virus (AAV) injection with the goal of enhancing virus uptake by targeting injections to neuromuscular junctions with electrical stimulation. We also describe procedures for non-invasively assessing functional opsin expression over time with transdermal optical stimulation of opsin-labeled nerves and electromyography (EMG) recordings. The presence of time-locked EMG spikes 4–8 ms after each stimulation pulse demonstrates that functional opsin expression is present at a given assessment time point. Onset of functional optical sensitivity generally occurs 2–4 weeks following virus injection, and sensitivity generally peaks or plateaus between 6–10 weeks. Stimulation sequences such as light intensity, stimulation pulse width, and frequency sweeps provide further information on functional opsin expression at the testing timepoint. The methods presented here can be used for driving functional opsin expression with a standard AAV6 vector commonly used in similar experiments or as a protocol for assessing peripheral nerve opsin expression with novel viral vectors.
Key features
• Uses electrical stimulation to guide needle placement during intramuscular viral injection.
• Drives robust and muscle-specific opsin expression in peripheral motor neurons.
• Describes transdermal optical stimulation sequences with varying stimulation light intensity, pulse width, and frequency for longitudinal assessment of opsin expression.
• Adaptable for use with multiple viral vectors and target muscles.
Keywords: Optogenetics (光遗传学)Graphical overview
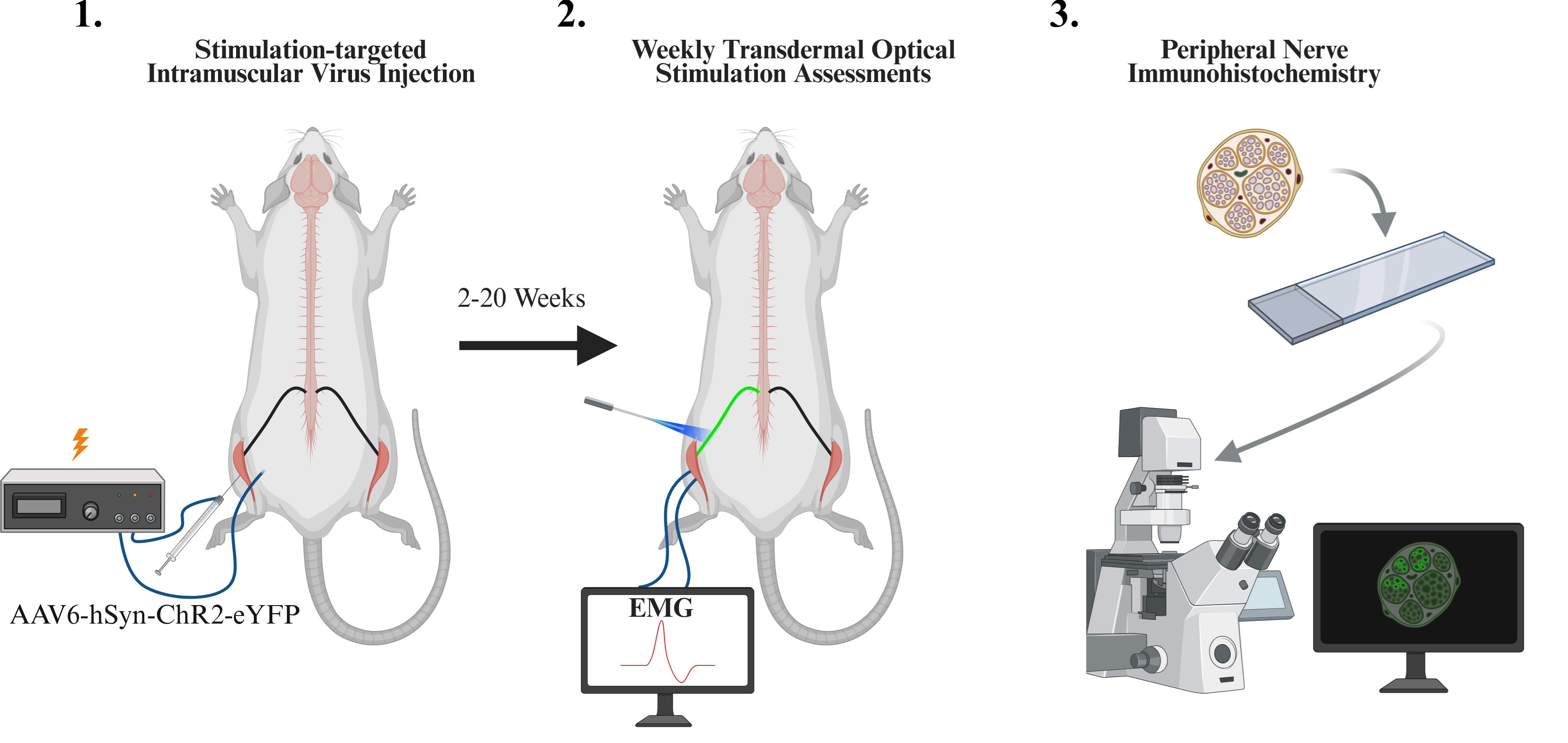
Overview of experimental protocol including intramuscular viral injection, weekly optical stimulation sessions, and immunohistochemistry
Background
Optogenetic stimulation or inhibition of peripheral motor nerves has recently emerged as an approach to directly modulate muscle activity in animal models from rodents to non-human primates [1–4]. In this approach, targeted nerves are genetically modified with light-gated ion channels (“opsins”) such as the common channel-rhodopsin2 (ChR2), allowing a nerve to be stimulated or inhibited with specific light wavelengths. Compared to electrical stimulation, this approach has been found to confer benefits, including a more physiological recruitment order of motor units (i.e., small muscle fibers are first recruited with low-intensity optical stimulation, and larger muscle fibers are added at higher intensities) and reduced stimulation-induced muscle fatigue [1,5]. Inhibition of target activity may be achieved using light-sensitive chloride channels or proton pumps (e.g., NpHR3 [6], ArchT [7]), a feat that is more complex with electrical stimulation. Additionally, stimulation of specific downstream nerve targets may be achieved through targeted virus injection, genetic targeting, or opsin selection [8,9]. Taken together, these traits present attractive benefits for the use of this technology in neuroscience or motor rehabilitation applications.
A key step for using this technology is to first biologically label target nerve axons with the desired opsin. Transgenic animals may be used for robust opsin labeling of genetically specified tissues throughout the body, including peripheral nerves for neuromodulation [1,10]. However, transgenic approaches are typically limited to mice, and specificity through these approaches is usually defined by genetic markers, resulting in the labeling of all tissues in the body that meet genetic criteria. Conversely, injection of viral vectors (e.g., adeno-associated virus, AAV) may be used to express optogenetic proteins targeted to tissues based on 1) the relative tropism of the virus (i.e., AAV3 for inner ear cells [11]), 2) genetic specificity tailored by the virus’s expression cassette, and 3) viral biodistribution guided by the route of virus delivery (e.g., broad expression via intravenous injection, focal expression via microinjection in the targeted tissue). These tools provide several knobs through which to tune the specificity or breadth of opsin expression.
In the case of peripheral optogenetic stimulation of motor function, muscle-specific opsin expression and stimulation can be achieved via intramuscular virus injection. For example, intramuscular injection of an AAV6 vector (e.g., AAV6-hSyn-ChR2-eYFP) into the tibialis anterior (TA) muscle of rats can be used to selectively label nerve axons innervating the TA with a light-sensitive opsin (e.g., channel-rhodopsin, ChR2) and a fluorescent tag (e.g., YFP) [2,3]. The human synapsin promoter (hSyn) restricts expression to neural tissue (as opposed to expression in the injected muscle). To label targeted deep peroneal nerve axons innervating just the TA muscle, a primary route would require that virus first crosses from the muscle into the nerve via the neuromuscular junction (NMJ), travel retrogradely up the nerve to motor neurons in the spinal cord, undergo transcription of the viral DNA instructions, and traffic expressed opsin and fluorescent proteins anterogradely back down the nerve axons. Following this injection route, optical stimulation at proximal nerve locations with multiple downstream muscle targets, such as the sciatic nerve, results in stimulation of only the targeted TA muscle [3], a feat of specificity that is challenging with electrical nerve stimulation.
A critical point in this viral transduction pathway is the uptake of the virus from the muscle into the nerve. NMJs are typically arranged in characteristic patterns within a muscle where nerve endings branch out to interface with muscle fibers. Targeting microinjections of virus to follow these NMJ distributions has been shown to increase the uptake of virus and other tracers [12,13]. While injection sites within a muscle may be based on expected NMJ distributions, intersubject variability could potentially limit the success of this approach, especially in larger animals. As an alternative approach, low-level electrical stimulation through intramuscular injection electrodes can be used to localize motor endplates in target muscles. Such an approach has been utilized in human subjects for guiding injections of botulinum toxin, in conditions such as juvenile cerebral palsy, as a fast and minimally invasive way to confirm injection of the targeted muscle and facilitate the paralytic effects of the injection [14,15]. We have previously employed a similar approach for intramuscular AAV injections in non-human primates to achieve optical stimulation of muscle activity [4,16].
Here, our primary purpose is to present a protocol to target AAV injections to motor endplates in hindlimb muscles of rats using electrical stimulation in order to express light-sensitive opsins along their corresponding nerves, thereby allowing light-based stimulation of muscle activity. Following these injections, we describe our common procedures for periodically evaluating a nerve’s optical sensitivity using transdermal laser stimulation to estimate and track the longitudinal time course of functional opsin expression. Finally, we present our standard histology preparations for examining the underlying patterns of opsin expression in nerve and spinal cord samples at the terminal end point of an experiment. These protocols provide a reliable means of achieving robust opsin expression in peripheral motor nerves and optically modulated motor responses. Additionally, these protocols can act as a framework for evaluating the functional opsin expression timelines and histological characteristics of optogenetic vectors with varying serotypes, promoters, and opsins.
Materials and reagents
Biological materials
1. Fischer rat (Charles River, strain 403, 100–200 g, generally 6–8 weeks, male or female)
2. AAV6-hSyn-ChR2(H134R)-EYFP [Virovek, 2E + 13 viral genomes per mL (vg/mL) in 1× PBS buffer containing 0.001% pluronic F-68 and 0.22 μm filter sterilized; storage: -80 °C, maximum one freeze-thaw cycle]
Reagents
1. Fast Green FCF (Fisher Scientific, catalog number: BP123-10), storage: 25 °C
2. Sterile water for injection (Fresenius Kabi, catalog number: 918510), storage: 25 °C
3. Loxicom (meloxicam) injection, 5 mg/mL (Norbrook Laboratories, catalog number: NDC 55529-040-10), storage: 25 °C
4. Povidone iodine prep solution (Dynarex Corporation, catalog number: NDC 67777-141-50), storage: 25 °C
5. Beuthanasia-D Special pentobarbital sodium and phenytoin sodium injection solution (Intervet Inc, catalog number: NDC 0061-0473-05), storage: 25 °C
6. OCT compound (Tissue-Tek, catalog number: 4583), storage: 25 °C
7. Molecular Probes ProLong Diamond Antifade Mountant (Fisher Scientific, catalog number: P36961), storage: 4 °C
8. Phosphate-buffered saline (PBS) (Midwest Scientific, catalog number: QS1200), storage: 25 °C
9. 4% paraformaldehyde (PFA) (Electron Microscopy Sciences, catalog number: 157-4), storage: 25 °C
10. Sodium azide (Sigma-Aldrich, catalog number: S2002), storage: 25 °C
11. Sucrose (Sigma-Aldrich, catalog number: S7903), storage: 25 °C
12. Rabbit anti-GFP antibodies (Thermo Fisher Scientific, catalog number: A11122), storage: 4 °C
13. Donkey anti-rabbit antibodies, AF647 conjugated (Jackson ImmunoResearch, catalog number: 711-607-003), storage: -80 °C
14. Normal donkey serum (Sigma-Aldrich, catalog number: D9663), storage: -20 °C
15. Triton X-100 (Thermo Fisher Scientific, catalog number: A16046.AP), storage: 25 °C
Laboratory supplies
1. Screw-cap microtubes, 1.5 mL, skirted (Thermo Fisher Scientific, catalog number: 3467-11)
2. Screw-cap microtubes, 0.5 mL, skirted (Thermo Fisher Scientific, catalog number: 3465)
3. Sterile syringe filters, 0.22 μm pore size, mixed cellulose ester membrane (Sigma-Aldrich, catalog number: SLGL0250S)
4. Disposable subdermal needle electrode, XS (Technomed, catalog number: TE/S50718-002; for EMG recording)
5. Disposable hypodermic needle electrode (Technomed, catalog number: TE/L2530-335; for stimulation during virus injection)
6. Covidien monoject hypodermic needles with aluminum hub (Covidien, catalog number: 8881200433; ground connection for EMG recording)
7. ETL Series: single alligator clips (The Electrode Store, catalog number: ETL-36BSAF; ground connection for EMG recording)
8. SGE gas-tight Teflon luer lock syringe, 50 μL (World Precision Instruments, catalog number: SGE050TLL)
9. Microscope cover glass (Midwest Scientific, catalog number: 1415-15)
10. Disposable base mold (Electron Microscopy Sciences, catalog number: 62352-07)
11. Tissue Path Superfrost Plus Gold slides (Fisher Scientific, catalog number: 1518848)
12. Animal feeding needle, 20 G straight (Pet Surgical, catalog number: AFN2025S; for perfusion)
Equipment
1. Micropositioner and magnetic stand (World Precision Instruments, catalog number: 1350M)
2. MICRO2T SMARTouch pump controller (World Precision Instruments, catalog number: MICRO2T)
3. UMP3 syringe pump (World Precision Instruments, catalog number: UMP3)
4. Nexus optical breadboard (Thorlabs, catalog number: B1824F)
5. LRD-0470 Collimated diode laser system, typical maximum output 200–250 mW at 470 nm, or 600 mW/mm2 at 1 mm from 200 μm, 0.22 NA optical fiber–based on a near-field approximation (Laserglow Technologies, catalog number: D4B2003FX)
6. Optical fiber, 200 μm, 0.22 NA FC/PC-FC/PC fiber patch cable (Thorlabs, catalog number: M122L05)
7. Optical power and energy meter console (Thorlabs, catalog number: PM400)
8. Slim photodiode power sensor (Thorlabs, catalog number: S130C)
9. Electrophysiology system with EMG recording and electrical stimulation capabilities (e.g., Tucker-Davis Technologies, RZ5D, SI-4, and S-Box)
10. Custom 3D-printed leg support (design available upon request)
11. Peristaltic pump (Kamoer, catalog number: KK1800)
12. Precision pump tubing, peroxide-cured silicone (Masterflex, catalog number: 96400-16)
13. Polycarbonate Luer-to-Barb adapter 1/8” hose barb (any)
14. Confocal microscope (e.g., Leica Microsystems, model: TCS SP8)
Procedure
文章信息
稿件历史记录
提交日期: Aug 29, 2025
接收日期: Nov 2, 2025
在线发布日期: Nov 23, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Moravec, E. M. and Williams, J. J. (2025). Stimulation-Guided AAV Delivery and Longitudinal Assessment of Optogenetic Expression in Rat Motor Nerves. Bio-protocol 15(24): e5545. DOI: 10.21769/BioProtoc.5545.
分类
神经科学 > 基础技术 > 光遗传学
微生物学 > 异源表达系统 > 腺相关病毒
神经科学 > 基础技术 > 肌电图
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











