- EN - English
- CN - 中文
Methods for Collecting and Analyzing Post-Ejaculatory Uterine Fluid and the Uterus in Mice
小鼠射精后子宫腔液及子宫组织的采集与分析方法
发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5544 浏览次数: 558
评审: Anonymous reviewer(s)
Abstract
In mammals, the semen is ejaculated into the female reproductive tract, and the sperm travel to the oviduct to fertilize the egg. A comprehensive understanding of the pre- and post-ejaculatory intrauterine environment is one of the key points for overcoming infertility; however, the dynamics of the intrauterine environment and its physiological role in the uterus, namely in the internal fertilization process, remain unclear. Conventional methods for collecting uterine fluids from the uterus post-ejaculation of mice show challenges regarding the ambiguous ejaculation timing. Here, we established a method for a mating environment with exact ejaculation timing. We also created a simple method for collecting pre- and post-ejaculatory uterine fluid without using forceps. Our methods achieved time-dependent biochemical and histological analyses of uterine fluids to provide fundamental information regarding protein composition and uterine structure changes during pre- and post-ejaculation. This protocol is suitable for analyzing temporal changes in reproductive phenomena, thereby contributing to elucidating the physiological role of the uterus in the process of intrauterine fertilization.
Key features
• This protocol is used for the simple collection of pre- and post-ejaculatory uterine fluid.
• Changes in the pre- and post-ejaculatory intrauterine environment can be examined by controlling the dissection time of females after ejaculation.
• An estrous female can be determined without a vaginal smear test in this protocol.
• This protocol can be used to analyze the protein composition of post-ejaculatory uterine fluid and is applicable to analyze sperm within the uterus post-ejaculation.
Keywords: Mating (交配)Graphical overview
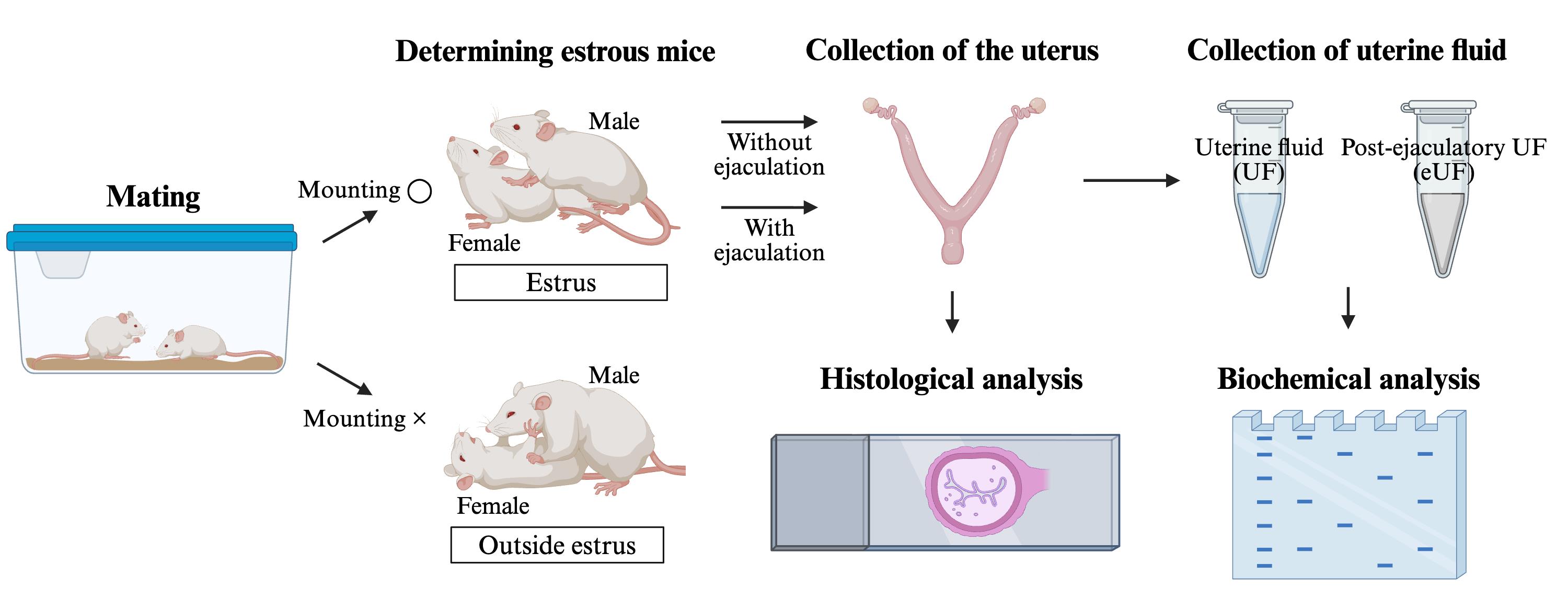
Methods for collecting and analyzing post-ejaculatory uterine fluid and the uterus
Background
The female reproductive tract comprises the vagina, uterus, oviducts, and ovaries. Mammals reproduce by internal fertilization, whereby the male ejaculates semen into the uterus or vagina. Ejaculated sperm fertilize eggs through a complex process in the female reproductive tract [1]. Mice, which share mechanistic features with humans in reproductive phenomena such as internal fertilization and implantation, are extremely useful as model animals. Previous studies in mice have reported that regulation of sperm migration in the uterotubal junction (UTJ) and sperm storage and capacitation in the oviduct are essential subprocesses of fertilization [2–4]. Although a recently published article suggested that an abnormal fluid environment within the post-ejaculatory uterus contributes to infertility [5], the role of the post-ejaculatory uterus in internal fertilization remains unclear compared to the UTJ and oviduct. To address this issue, it is necessary to establish protocols that allow evaluation of pre-ejaculatory, post-ejaculatory (immediately post-ejaculation or pre-fertilization), and post-ejaculatory (post-fertilization) intrauterine environment. The conventional protocol for collecting post-ejaculatory uterine fluid (eUF) from the uterus post-ejaculation is limited in that it cannot collect eUF immediately after post-ejaculation. Therefore, we propose a simple method for collecting pre-and post-ejaculatory (immediately post-ejaculation) uterine fluid and uterus, followed by biochemical and histological analyses. This protocol provides a novel approach to study dynamics of the uterus, sperm (motility, capacitation, and survival), and fertilization in internal fertilization, which is expected to contribute to elucidating the physiological role of the uterus.
Materials and reagents
Biological materials
1. ICR male and female mice (Japan SLC, Inc.)
Reagents
1. Ethanol 70% (Yoshida Pharmaceutical Company, Ecosyoueta Disinfectant Solution, catalog number: 14987288980046)
2. PierceTM bovine serum albumin standard ampules, 2 mg/mL (Thermo Fisher Scientific, catalog number: 23209)
3. PierceTM BCA Protein Assay kit (FUJIFILM Wako Pure Chemical Corp., catalog number: 297-73101)
4. NuncTM MicroWellTM 96-well, Nunclon Delta-treated, flat-bottom microplate (Thermo Fisher Scientific, catalog number: 167008)
5. 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M314835406-91)
6. Sodium dodecyl sulfate (SDS) (FUJIFILM Wako Pure Chemical, catalog number: 191-07145)
7. Glycerol (FUJIFILM Wako Pure Chemical, catalog number: 075-00616)
8. Bromophenol blue (FUJIFILM Wako Pure Chemical, catalog number: 029-02912)
9. Tris(hydroxymethyl)aminomethane (Tris) (NACALAI TESQUE, Inc., catalog number: 35406-91)
10. Hydrochloric acid (HCl) (FUJIFILM Wako Pure Chemical, catalog number: 080-01066)
11. Acrylamide/bis solution 37.5:1 at 30% (Bio-Rad Laboratories, Inc., catalog number: 1610158)
12. N,N,N',N'-Tetramethylethylenediamine (TEMED) (FUJIFILM Wako Pure Chemical, catalog number: 205-06313)
13. Ammonium persulfate (APS) (FUJIFILM Wako Pure Chemical, catalog number: 802811)
14. Urea (FUJIFILM Wako Pure Chemical, catalog number: 219-00175)
15. Glycine (FUJIFILM Wako Pure Chemical, catalog number: 077-00735)
16. Protein molecular weight marker (FUJIFILM Wako Pure Chemical, catalog number: 234-02464)
17. Coomassie brilliant blue R-250 (CBB-R250) (Thermo Fisher Scientific, catalog number: 20278)
18. Acetic acid (FUJIFILM Wako Pure Chemical, catalog number: 017-00251)
19. Methanol (FUJIFILM Wako Pure Chemical, catalog number: 131-01826)
20. 10× Phosphate-buffered saline (PBS) (TOHO Co., Ltd., catalog number: 12-9423-5)
21. Bouin's solution (FUJIFILM Wako Pure Chemical, catalog number: 023-17361)
22. Sucrose (FUJIFILM Wako Pure Chemical, catalog number: 196-00015)
23. Tissue-Tek® Cryomold® (Sakura Finetek Japan Co., Ltd., catalog number: 4557)
24. Optimal cutting temperature (OCT) compound (Sakura Finetek Japan, catalog number: 45833)
25. Liquid nitrogen
26. New hematoxylin solution type M (Muto Pure Chemicals, catalog number: 30142)
27. Eosin Y solution at 1% (Muto Pure Chemicals, catalog number: 32002)
28. Ethanol 100% (Muto Pure Chemicals, catalog number: 43105)
29. Xylene (Muto Pure Chemicals., catalog number: 43122)
30. Mounting medium (New M·X) (Matsunami Glass Industry Co., Ltd., catalog number: FX00500)
Solutions
1. Tris/HCl (pH 6.8, pH 8.8), 1 M (see Recipes)
2. 6× Sample buffer (see Recipes)
3. SDS 10% (see Recipes)
4. APS 25% (see Recipes)
5. Stacking gel solution (see Recipes)
6. Separation gel solution (see Recipes)
7. Running buffer (see Recipes)
8. CBB staining solution (see Recipes)
9. CBB decolorizing solution (see Recipes)
10. Eosin solution (see Recipes)
Recipes
1. Tris/HCl (pH 6.8, pH 8.8), 1 M
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris | 1 M | 60.57 g |
| Adjust to pH 6.8 and pH 8.8 with HCl | ||
| Distilled water | n/a | <500 mL |
| Total | n/a | 500 mL |
Note: pH 6.8 and pH 8.8 Tris/HCl solutions are prepared separately.
2. 6× sample buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris/HCl (pH 6.8), 1 M | 350 mM | 3.5 mL |
| 2-Mercaptoethanol | 30% (v/v) | 3 mL |
| SDS | 10% (w/v) | 1 g |
| Glycerol | 30% (v/v) | 3 mL |
| Bromophenol blue | 0.06% (w/v) | 6 mg |
| Distilled water | n/a | 0.5 mL |
| Total | n/a | 10 mL |
3. SDS 10% (w/v)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| SDS | 10% (w/v) | 20 g |
| Distilled water | n/a | <200 mL |
| Total | n/a | 200 mL |
4. APS 25% (w/v)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| APS | 25% (w/v) | 2.5 g |
| Distilled water | n/a | <10 mL |
| Total | n/a | 10 mL |
5. Stacking gel solution
| Reagent | Quantity or volume |
|---|---|
| 30% Acrylamide/bis solution 37.5:1 | 375 μL |
| Tris/HCl (pH 6.8), 1 M | 468.75 μL |
| SDS, 10% (w/v) | 37.5 μL |
| TEMED | 3 μL |
| Distilled water | 2856.25 μL |
| Total | 3740.6 μL |
6. Separation gel solution
| Reagent | Quantity or volume |
|---|---|
| 30% Acrylamide/bis solution 37.5:1 | 2000 μL |
| Tris/HCl (pH 8.8), 1 M | 2812.5 μL |
| SDS, 10% (w/v) | 75 μL |
| TEMED | 6 μL |
| Distilled water | 2587.5 μL |
| Total | 7481 μL |
7. Running buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris | 25 mM | 3.03 g |
| SDS | 0.1% (w/v) | 1 g |
| Glycine | 192 mM | 14.4 g |
| Distilled water | n/a | <1,000 mL |
| Total | n/a | 1,000 mL |
8. CBB staining solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CBB R-250 | 0.25% (w/v) | 0.5 g |
| Acetic acid | 10% (v/v) | 20 mL |
| Methanol | 50% (v/v) | 100 mL |
| Distilled water | n/a | 80 mL |
| Total | n/a | 200 mL |
9. CBB decolorizing solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetic acid | 7.5% (v/v) | 15 mL |
| Methanol | 25% (v/v) | 50 mL |
| Distilled water | n/a | 135 mL |
| Total | n/a | 200 mL |
10. Eosin solution
| Reagent | Quantity or Volume |
|---|---|
| 1% Eosin Y solution | 100 mL |
| 60% ethanol | 500 mL |
| Acetic acid | 0.6 mL |
| Total | 600.6 mL |
Laboratory supplies
1. Disposable latex gloves (ASKUL, catalog number: WE59721)
2. Microcentrifuge tubes 1.5 mL (Greiner Bio-One Co., Ltd., catalog number: 616201)
3. Centrifuge tubes 15 mL (AS ONE, catalog number: 4-3632-01)
4. Pipette tips 10 μL (WATSON, catalog number: 110-207C)
5. Pipette tips 200 μL (WATSON, catalog number: 110-705C)
6. Pipette tips 1,000 μL (WATSON, catalog number: 110-7-6C)
7. Gel loading tips 200 μL (BM Equipment Co., Ltd., catalog number: 010-Q)
8. Kimwipes (NIPPON PEPAR CRECIA Co., Ltd., catalog number: 62020)
9. Paper towels (ASKUL, catalog number: 1944368)
10. Dish 60 mm in size (AGC TECHNO GLASS Co., Ltd., catalog number: 1010-060)
11. Absorbent paper (ATTO Corp., catalog number: CB-06A)
12. Glass slide (Muto Pure Chemicals Co., Ltd., catalog number: 513617)
13. 24 mm × 50 mm cover glass (thickness: 0.13–0.17 mm) (Matsunami Glass Industry, catalog number: C024501)
Equipment
1. Personal protective equipment (e.g., mask, goggles, and lab coats)
2. Precision balance (Mettler Toledo, model: PB602-S)
3. P-20 pipette (Gilson, model: F123600)
4. P-200 pipette (Gilson, model: F123601)
5. P-1000 pipette (Gilson, model: F120602)
6. Small straight scissors (Natsume Seisakusho, model: B-12)
7. Large straight scissors (Natsume Seisakusho, model: B-3)
8. Tweezers (AS ONE, model: 2-529-12)
9. Plastic cages (Clea Japan, Inc., model: CL-0103-2 Mouse TPX)
10. Water bottles, rubber stoppers (Clea Japan, Inc., model CL-0904)
11. SpectraMax (Molecular Devices, model: SpectraMax® iD5e)
12. 1 mm dual mini gel cast (glass plates, seal gasket, comb, and clips) (ATTO, model: AE-6401)
13. pH meter (HIRIBA, model: F-72)
14. Beaker glass, 500 mL (AS ONE, model: 2-5091-06)
15. Magnetic stirrer (Thermo Fisher Scientific, model: Magnetic stirrer RT Basic-12)
16. Stirrer (AS ONE, model: 3-6657-02)
17. Electrophoresis system (ATTO, model: WSE-1100)
18. Rocking mixer (AS ONE, model: 1-5829-22)
19. Cryostat (Thermo Fisher Scientific, model: CryoStar NX50)
20. Microscope (Keyence Corp., model: BZ-X700)
Software and datasets
1. Microsoft Excel version 16.102.1 (Microsoft Corporation)
2. BioRender (https://www.biorender.com/)
Procedure
文章信息
稿件历史记录
提交日期: Sep 27, 2025
接收日期: Nov 16, 2025
在线发布日期: Nov 24, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Matsumoto, Y., Sato, B., Inui, M., Sunamoto, M., Kawano, N. and Miyado, K. (2025). Methods for Collecting and Analyzing Post-Ejaculatory Uterine Fluid and the Uterus in Mice. Bio-protocol 15(24): e5544. DOI: 10.21769/BioProtoc.5544.
分类
发育生物学 > 繁殖 > 生殖细胞
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












