- EN - English
- CN - 中文
Manufacturing of Living Building Materials With Calcifying Cyanobacteria
利用具钙化能力的蓝藻制备活体建筑材料
(*contributed equally to this work) 发布: 2025年12月20日第15卷第24期 DOI: 10.21769/BioProtoc.5543 浏览次数: 696
评审: Alba BlesaSrajan KapoorAnonymous reviewer(s)
Abstract
In recent years, the calcifying properties of some cyanobacteria have been used in the production of living building materials (LBMs), such as bio-concrete, as a CO2-friendly alternative for cement. This microbially induced calcium carbonate precipitation (MICP) technique can act as a novel platform technology for carbon capture strategies. Consequently, various research articles have been conducted based on a diverse set of workflows, including several modifications, to manufacture LBMs. However, such articles contain only fragmentary descriptions of the materials and methods used. This protocol is meant to act as a detailed, step-by-step operational manual for the production of LBMs using the cyanobacterial model strain Picosynechococcus sp. PCC 7002. The process is divided into several steps, such as the activation of the cyanobacterial-gel solution with CaCl2 × 2H2O and NaHCO3, casting standardized prisms (160 × 40 × 40 mm), and demolding LBMs. Subsequently, bending tensile and compressive strength tests are performed according to the procedures commonly used in concrete and material testing as proof of concept.
Key features
• A comprehensive workflow for the manufacturing of cement-free living building materials with cyanobacteria.
• A cyanobacteria-gelatin-containing solution is activated, mixed with sand, casted, curated, and strength tested.
• Adaptable for other cyanobacterial strains and substitute materials.
Keywords: Cement (水泥)Graphical overview
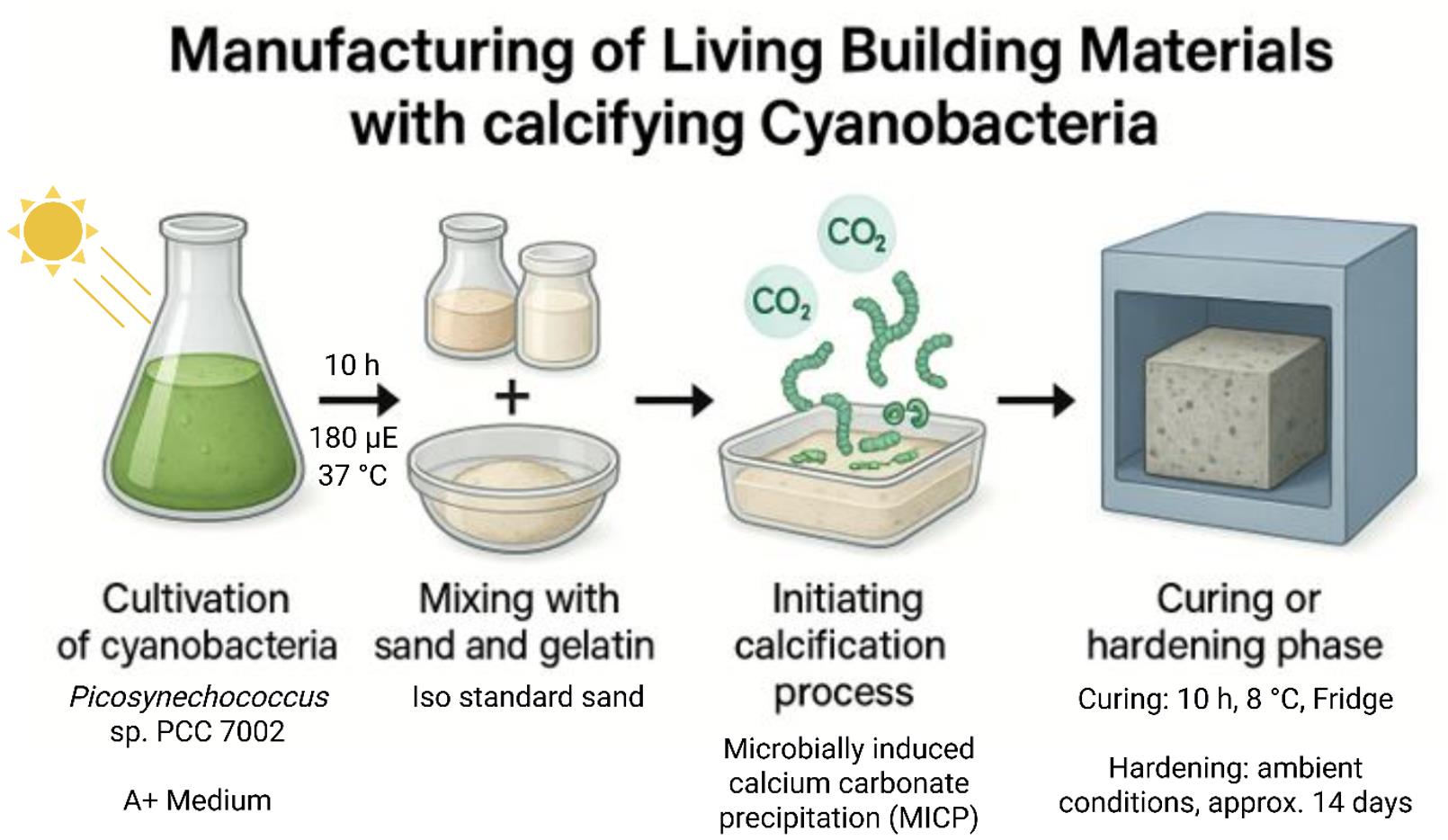
Simplified scheme for the manufacturing of living building materials (LBMs) such as concrete-like bricks based on the calcification properties of cyanobacteria
Background
In view of the ongoing global climate change and associated ecological and economic changes, innovations to reduce emissions are becoming increasingly important. Carbon dioxide (CO2) is the primary factor in this context and the key to preventing long-term, irreversible consequences. Over the last 20 years alone, annual CO2 emissions have risen by around 45% to an annual 37.12 billion tons [1]. The use of renewable energies and the development of new technologies are the main strategies for achieving the goals of the Paris Climate Agreement and thus a global temperature rise of no more than 1.5–2.0 °C by 2100 [2].
The construction industry plays a decisive role in this. In recent years, the trend shows that the manufacturing sector of the construction industry, in particular, has not been able to achieve any emission reductions [3]. One possible explanation for this could be that adequate substitutes have not yet been developed for the main components in the production of resource-intensive materials. For example, it is not yet possible to produce concrete without cement or entirely from recycled concrete.
Totaling 37% of all CO2 emissions, the building sector is one of the main producers of greenhouse gases in the construction industry. Of this, 10% is directly attributable to the construction industry. As a result, around 5%–8% of annual global CO2 emissions are attributable to the cement industry, which is therefore seen as a target for optimization. The development of alternative building materials and new recycling methods for more sustainable construction is therefore of great interest for the near future [4].
Research on cyanobacteria is interesting in this context due to their ability to fix CO2. During biomineralization, CO2 from the environment is bound and supports the precipitation of calcium carbonate (CaCO3) under certain circumstances as a consequence of their carbon concentration mechanism (CCM) [5]. This process has already been used to manufacture living building materials (LBMs), and thus, the combination of biotic additives with conventional building materials is currently being researched in order to completely replace cement with microbially induced calcification. Although this has successfully been achieved with cyanobacterial model strains such as Picosynechococcus sp. PCC 7002 [6,7], as additives for 3D printing [8] and using other cyanobacterial strains [9], a detailed, general, and comprehensive workflow is missing [10].
In order to provide an extensive methodological description of the workflow for the manufacturing of LBMs with cyanobacteria, we set up this protocol. It is divided into the following steps: i) preparation and incubation of the cyanobacteria-gel solution, ii) casting of the LBMs, iii) de-molding and curation of the LBMs, and iv) bending tensile- and compressive strength tests as a proof of concept. Our protocol describes the general process of manufacturing but also includes alternative steps to broaden the utilization of the protocol for future development of cyanobacterial LBMs.
Materials and reagents
Biological materials
1. Picosynechococcus sp. PCC 7002 from Pasteur Culture Collection [11]
Reagents
1. NaCl (Merck, CAS: 7647-14-5)
2. MgSO4·7H2O (Merck, CAS: 7487-88-9)
3. Na2EDTA·2H2O (Merck, CAS: 6381-92-6)
4. KCl (Merck, CAS: 7447-40-7)
5. CaCl2·2H2O ((Merck, CAS: 10035-04-8)
6. NaNO3 (Sigma-Aldrich, CAS: 7631-99-4)
7. KH2PO4 (Merck, CAS: 7778-77-0)
8. Trizma base (Merck, CAS: 77-86-1)
9. H3BO3 (Merck, CAS: 10043-35-3)
10. ZnCl2 (VWR Chemicals, CAS: 7646-85-7)
11. MoO3 (Merck, CAS: 1313-27-5)
12. Vitamin B12 (Merck, CAS: 68-19-9)
13. FeCl3·6H2O (Merck, CAS: 10025-77-1)
14. MnCl2·4H2O (Merck, CAS: 13446-34-9)
15. CuSO4·5H2O (Merck, CAS: 7758-99-8)
16. CoCl2·6H2O (Merck, CAS: 7791-13-1)
17. Gelatin (VWR, CAS: 24350.262)
18. ISO standard sand (Normensand.de, CEN-Normsand EN196-1)
19. Oil (any virgin organic rapeseed oil)
20. HCl (Sigma-Aldrich, CAS: 7647-01-0)
21. NaOH (Honeywell Chemicals, CAS: 1310-73-2)
Solutions
1. A+ trace components [12] (see Recipes)
2. A+ medium [12] (see Recipes)
3. A+ modified medium [6] (see Recipes)
Recipes
1. A+ trace components [12]
| Component | Reagent | Stock solution (g/100 mL) | Nutrient solution |
|---|---|---|---|
| 1 | H3BO3 | 3.43 g/L | |
| 2 | ZnCl2 | 0.0315 g/L | |
| 3 | MoO3 | 0.003 g/L | |
| 4 | Vitamin B12 | 0.0004 g/L | |
| 5 | FeCl3·6H2O | 3.89 | 1 mL/L |
| 6 | MnCl2·4H2O | 4.3 | 1 mL/L |
| 7 | CuSO4·5H2O | 0.003 | 1 mL/L |
| 8 | CoCl2·6H2O | 0.012 | 1 mL/L |
a. To prepare 1 L of A+ trace components, add 500 mL of dH2O to a 1 L glass bottle and add 3.43 g of component 1, 0.0315 g of component 2, 0.003 g of component 3, and 0.0004 g of component 4 as described above while stirring continuously.
b. Prepare 100 mL of each stock solution from components 5–8. Use Milli-Q or dH2O for each solution.
c. Then, add 1 mL of each stock solution from components 5–8 above and adjust to 1 L by adding dH2O.
d. Store all stock solutions and A+ trace components in a refrigerator.
2. A+ medium [12]
| Component | Reagent | Stock solution (g/100 mL) | Nutrient solution |
|---|---|---|---|
| 1 | NaCl | 18 g/L | |
| 2 | MgSO4·7H2O | 5 g/L | |
| 3 | Na2EDTA·2H2O | 0.3 | 10 mL/L |
| 4 | KCl | 6 | 10 mL/L |
| 5 | CaCl2·2H2O | 3.7 | 10 mL/L |
| 6 | NaNO3 | 10 | 10 mL/L |
| 7 | KH2PO4 | 0.5 | 10 mL/L |
| 8 | Trizma base pH 8.2 | 10 | 10 mL/L |
| 9 | A+ trace components | 10 mL/L |
a. Prepare 100 mL of each stock solution from components 3–8 from the table above. Use Milli-Q or dH2O for each solution.
b. To prepare 1 L of A+ medium, add 500 mL of dH2O in a 1 L glass bottle and add 18 g of component 1, 5 g of component 2, and 10 mL of each stock solution of components 3–8 as shown above, while stirring continuously.
Note: Store all stock solutions in the dark at room temperature.
c. Fill up to 1 L with dH2O and adjust pH to 7.6 with HCl solution.
d. Sterilize by autoclaving (121 °C, 15 psi, 20 min).
e. Add 10 mL of A+ trace components (see Recipe 1) by filter sterilization (0.22 μm).
Notes:
1. A+ trace components is heat sensitive.
2. For agar plates, A+ medium is supplemented with sodium thiosulfate pentahydrate (24.819 g/mL) stock solution; 1 mL/L A+ medium and 1.5% (w/v) agarose.
f. Store the final A+ medium at room temperature before usage.
3. A+ modified medium [6]
| Component | Reagent | Stock solution (g/100 mL) | Nutrient solution |
|---|---|---|---|
| 1 | MgSO4·7H2O | 5 g/L | |
| 2 | Na2EDTA·2H2O | 0.3 | 10 mL/L |
| 3 | KCl | 6 | 10 mL/L |
| 4 | CaCl2·2H2O | 3.7 | 10 mL/L |
| 5 | NaNO3 | 10 | 10 mL/L |
| 6 | KH2PO4 | 0.5 | 10 mL/L |
| 7 | Trizma base pH 8.2 | 10 | 10 mL/L |
| 8 | A+ trace components | 10 mL/L |
Note: NaCl is left out to avoid halite formation.
Laboratory supplies
1. Pipette tips with filter (Mettler Toledo, model: RAININ)
2. Laboratory bottle, 1 L (VWR, catalog number: 215-1517P)
3. Magnetic stirring bar (VWR Collection, catalog number: 442-4521)
4. Sterile indicator strip (Roth, catalog number: XC20.1)
5. Sterile filter 0.22 μm (VWR Collection, catalog number: 514-1266)
6. Plastic syringe 10 mL (Henke Sass Wolf, catalog number: HSWA8300063477)
7. Plastic bottles 50 mL (VWR collection, catalog number: 734-0448)
8. Metal bowls (Roth, catalog number: L942.1)
9. Trowel (Hornbach, catalog number: 8098631)
10. Glass beaker (VWR Collection, catalog number: 213-0480)
11. Nitrile gloves (VWR, catalog number: ANSE93-143/8.5-9)
12. Lids with two olives (VWR Collection, catalog number: SCOT293102807; SCOT1129825)
13. Silicon pipes (Freudenberg Medical Europe GmbH, catalog number: 228-1069/228-1070)
14. Check valves (Brand, catalog number: 229-3311)
15. Spatula (VWR Collection, catalog number: RSGA395.150)
Equipment
1. Pipettes (Mettler Toledo, model: Pipet-Lite XLS series)
2. Magnetic stirrer/heat function (VWR Collection, catalog number: 442-0661)
3. Autoclave (Fedegari FVG, model: FVG3)
4. Centrifuge (Sigma, catalog number: SIGMA-22017)
5. Clean safety bench (Fedegari FVG, NuAire, model: NU-543)
6. Scientific scales (Sartorius, model: 1712)
7. Precision three-gang molds (form+test prüfsysteme, model: B2709)
8. Fridge (KBS, catalog number: DKU2031)
9. Universal testing machine (ZwickRoell, model: Z100)
10. NanoDrop One (ThermoFisher Scientific, catalog number: ND-ONE-W)
11. Culture cabinet (CLF PlantClimatics/Percival)
12. Full spectral light (MICCYE, model: B0D97RYTGZ)
13. Digital pH measurement device (Mettler Toledo, catalog number: 30046242)
14. Brush (MKK Pinsel, catalog number: 20387)
Procedure
文章信息
稿件历史记录
提交日期: Aug 26, 2025
接收日期: Nov 12, 2025
在线发布日期: Nov 21, 2025
出版日期: Dec 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Jung, P., Friedek, J., Briegel-Williams, L., Haage-Ott, M. and Neff, C. (2025). Manufacturing of Living Building Materials With Calcifying Cyanobacteria. Bio-protocol 15(24): e5543. DOI: 10.21769/BioProtoc.5543.
分类
生物工程
植物科学 > 植物生理学 > 生物矿化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









