- EN - English
- CN - 中文
Preparation and Negative Staining for Visualization of Cyanoglobule Lipid Droplets Using Transmission Electron Microscopy
利用透射电镜观察蓝细菌胞蓝小体脂滴的制备与负染方法
发布: 2025年12月05日第15卷第23期 DOI: 10.21769/BioProtoc.5532 浏览次数: 1311
评审: Samik BhattacharyaDawid S ZylaAnonymous reviewer(s)
Abstract
Lipid droplets have emerged as dynamic organelles involved in diverse cellular processes beyond simple lipid storage. In plants and cyanobacteria, growing evidence highlights their importance in stress adaptation and signaling, yet methods to study their structure and purity remain limited. Traditionally, in situ transmission electron microscopy (TEM) has been used to visualize lipid droplets within intact cells. While powerful, this approach cannot easily evaluate isolated lipid droplets or confirm their purity. In this protocol, we describe a rapid method for preparing and visualizing cyanoglobule lipid droplets isolated from cyanobacteria. The isolated droplets are directly processed for TEM using negative staining with uranyl acetate, providing a straightforward and efficient workflow. The procedure can be applied broadly to lipid droplets from diverse organisms, independent of species or cellular origin. This protocol offers a simple, fast, and widely applicable approach to assessing lipid droplets, expanding the toolkit for researchers studying their structure and function.
Key features
• Provides a brief and detailed method to visualize cyanoglobule lipid droplets.
• Offers a rapid workflow (~1–2 h from preparation to imaging), enabling efficient sample processing and high-throughput analysis.
• Employs negative staining and TEM to directly assess droplet morphology and purity without complex sample preparation.
• Applicable to isolated lipid droplets from diverse organisms, making it a broadly useful tool beyond cyanobacteria.
Keywords: Lipid droplets (脂滴)Graphical overview
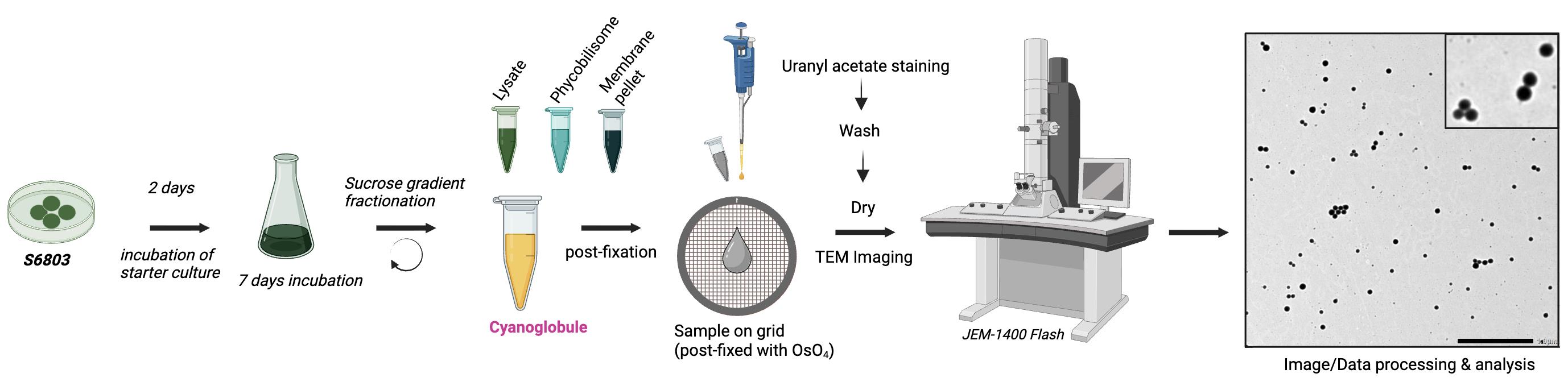
Overview of cyanoglobule lipid droplet preparation and negative staining for transmission electron microscopy (TEM) visualization
Background
Plastoglobules are lipid–protein droplets in chloroplasts that play important roles in membrane remodeling, stress adaptation, and redox balance [1,2]. They are closely associated with thylakoid membranes and change dynamically in number and size in response to stress [3]. Proteomic studies in Arabidopsis thaliana have revealed a specialized set of enzymes involved in lipid metabolism, redox regulation, and hormone biosynthesis, supporting the view that plastoglobules function as metabolic platforms rather than passive storage sites [4–7]. Despite these insights, knowledge of plastoglobule lipid and protein composition remains limited outside of model systems.
In cyanobacteria, various inclusion bodies such as carboxysomes, glycogen granules, and polyhydroxy alkanoate (PHA) bodies are well studied, while lipid droplets have only recently attracted attention. These structures, now termed cyanoglobules, are widely observed across species and increase in abundance under nutrient stress conditions [8–10,16]. Early studies suggested they contained triacylglycerol, though recent work has shown that they also harbor unique plastoquinone derivatives [11,12,16]. Notably, the lipid composition of cyanoglobules can vary depending on environmental stress and species, reflecting their dynamic metabolic roles. Proteomic and lipidomic analyses indicate that cyanoglobules share core features with plastoglobules, including enrichment in prenyl lipids and conserved proteins, while also exhibiting unique attributes such as stress-induced carotenoid accumulation [2,7,16]. These findings suggest that cyanoglobules are metabolically active compartments with important roles in stress physiology and share an evolutionary relationship with plastoglobules [13,14,16].
Traditionally, plastoglobules and cyanoglobules have been studied using in situ transmission electron microscopy (TEM), which provides high-resolution imaging within the cellular context but does not readily allow evaluation of isolated lipid droplets or assessment of sample purity [15]. Biochemical methods such as thin-layer chromatography (TLC) or gas chromatography–mass spectrometry (GC–MS) have advanced our understanding of lipid composition, but they do not provide rapid or direct visualization of droplet morphology. Negative staining TEM of isolated droplets offers a valuable complementary approach, enabling direct imaging of droplet morphology, size, and purity with minimal preparation.
Here, we present a rapid and broadly applicable protocol for visualizing cyanoglobule lipid droplets by negative staining TEM. Unlike conventional resin embedding and thin-section TEM, which require time-consuming fixation, dehydration, and sectioning steps that may alter droplet morphology, negative staining enables direct observation of isolated structures in near-native form. This approach provides a straightforward workflow to directly assess droplet morphology and integrity immediately after isolation, thereby expanding the toolkit available for studying cyanobacterial lipid droplets. Beyond cyanobacteria, this method can also be applied to plastoglobules and potentially to cytosolic lipid droplets in diverse organisms. Its simplicity, speed, and reproducibility make it a powerful method for advancing the study of lipid droplet biology, stress adaptation, and evolutionary connections across the photosynthetic lineage.
Materials and reagents
Biological materials
1. Isolated Synechocystis sp. PCC 6803 cyanoglobule lipid droplets (prepared as described previously [17])
Reagents
1. Osmium tetroxide (OsO4) 4% (Electron Microscopy Sciences, catalog number: 19140)
2. 1% uranyl acetate (Electron Microscopy Sciences, catalog number: 22400-1)
Note: The uranyl acetate working solution should be stored at 4 °C, protected from light, for up to one month. Before use, filter the solution through a 0.22 μm syringe filter to remove particulates and ensure optimal staining quality.
3. HPLC-grade distilled water (Sigma-Aldrich, catalog number: 270733)
Solutions
1. 1% osmium tetroxide (see Recipes)
Recipes
1. 1% osmium tetroxide (OsO4)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| OsO4 4% (w/v) | 1% (w/v) | 2.5 mL |
| HPLC-grade distilled water | n/a | 7.5 mL |
| Total | 10 mL |
Note: OsO4 is extremely toxic and volatile, so always prepare and handle it in a certified fume hood, with gloves and eye/face protection. Store in sealed glass vials at 4 °C, under refrigeration in secondary containment. Vials should display appropriate universal hazard symbols according to local environmental, health, and safety guidelines.
Laboratory supplies
1. Parafilm (Heathrow Scientific, catalog number: 470201-930)
2. Formvar-coated grid (100 mesh) (Electron Microscopy Sciences, catalog number: FCF100-Cu)
3. Micropipette
4. P1000, P200, P10 pipette tips
5. Petri dish (glass) (VWR, catalog number: 75845-542)
6. Forceps (anti-capillary) (Electron Microscopy Sciences, catalog number: 72700-D)
7. Filter paper (VWR, catalog number: 470201-456)
Equipment
1. JEM-1400 Flash Electron Microscope, equipped with a Matataki EM-14661 Flash High Sensitive CMOS camera (sCMOS) (JEOL Ltd.)
Software and datasets
1. Operation GUI (TEM Center version 1.7.16) (JEOL Ltd)
Procedure
文章信息
稿件历史记录
提交日期: Sep 3, 2025
接收日期: Nov 3, 2025
在线发布日期: Nov 13, 2025
出版日期: Dec 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Susanto, F. A., Withrow, A. and Lundquist, P. K. (2025). Preparation and Negative Staining for Visualization of Cyanoglobule Lipid Droplets Using Transmission Electron Microscopy. Bio-protocol 15(23): e5532. DOI: 10.21769/BioProtoc.5532.
分类
植物科学 > 植物细胞生物学 > 细胞器分离
微生物学 > 微生物细胞生物学 > 细胞器分离
细胞生物学 > 细胞器分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












