- EN - English
- CN - 中文
Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
重新优化原代小胶质细胞分离流程:改进的小胶质细胞提取方法
发布: 2025年12月05日第15卷第23期 DOI: 10.21769/BioProtoc.5530 浏览次数: 1475
评审: Elena A. OstrakhovitchEmmanuelle BerretAnonymous reviewer(s)

相关实验方案

基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1658 阅读
Abstract
Microglia, the resident immune cells of the central nervous system, play a crucial role in maintaining neural homeostasis and in regulating neurodevelopment, neuroinflammation, tissue repair, and neurotoxicity. They are also key contributors to the pathogenesis of various neurodegenerative disorders, underscoring the need for in vitro models that accurately recapitulate disease-relevant conditions. Among the available isolation methods, the classical mixed glial culture shaking technique remains the most commonly employed, while alternatives such as magnetic bead separation and fluorescence-activated cell sorting (FACS) offer higher purity but are often constrained by technical complexity and cost. In this study, we refined the traditional shaking method by supplementing specific cytokines during culture to enhance microglial viability and proliferation. Our optimized protocol produced primary microglia with higher purity, greater yield, and improved viability compared with the conventional approach, thereby increasing experimental efficiency while substantially reducing time, animal usage, and overall cost.
Key features
• The microglial cells obtained using this protocol achieve a purity of approximately 90%.
• This protocol maximizes the viability of primary microglial cells.
• The entire procedure requires a minimum of 9 days to complete.
• Antibiotic or antifungal solutions are not used in this protocol.
Keywords: Cell culture (细胞培养)Graphical overview
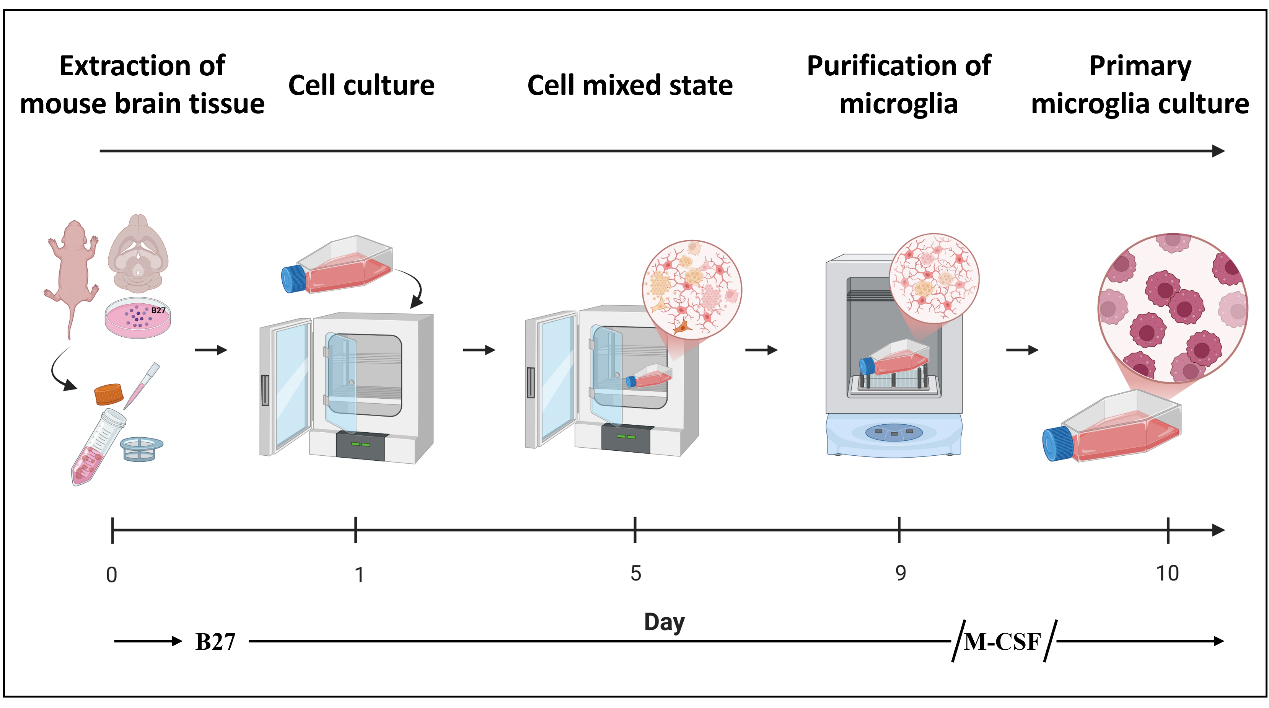
Flowchart of the isolation procedure for primary microglial cells from mice
Background
Microglia are the resident immune effector cells of the central nervous system (CNS), endowed with essential functions such as phagocytosis of pathogens and clearance of apoptotic cells. They are primarily responsible for maintaining neural homeostasis, mediating inflammatory responses, and contributing to tissue repair. Increasing evidence has highlighted their pivotal roles in pathological conditions, including neurodegenerative diseases, brain injury, and psychiatric disorders, which have become major focuses of contemporary neuroscience research [1–4]. Although several established in vitro models, such as the murine BV2 microglial cell line, are widely used, they exhibit intrinsic limitations as experimental systems [5]. In detail, the immortalization of BV2 cells may lead to aberrant gene expression, dysregulated signaling pathways, and altered metabolic states. Compared with primary microglia, BV2 cells lack key homeostatic features and immune functionalities, making them less suitable for accurately modeling the physiological responses of microglia in Parkinson’s disease (PD) [6]. Consequently, obtaining primary microglia with both high yield and high purity is of great importance for mechanistic studies in molecular neuroscience. Furthermore, in vitro experiments employing purified primary microglia provide a controllable and physiologically relevant model for dissecting activation pathways and intercellular interactions, thereby facilitating a deeper understanding of their functions under diverse pathological states.
Several approaches have been developed for the isolation of primary microglia from mice, including differential adherence, magnetic-activated cell sorting (MACS), fluorescence-activated cell sorting (FACS), and the mixed glial culture shaking method [7–9]. Among them, MACS and FACS offer high specificity and yield microglia of greater purity; however, these methods are limited by their high cost and relatively low overall recovery. In contrast, differential adherence and shaking methods are technically straightforward and cost-effective for routine use, yet they typically produce cells of lower purity. Therefore, developing an isolation protocol that allows rapid, efficient, and cost-effective acquisition of highly purified primary microglia would be highly valuable for experimental model establishment and subsequent mechanistic studies.
In this protocol, we optimized the traditional mixed glial culture shaking method for microglial isolation. During the initial brain tissue dissociation and early mixed glial culture stage, the neuronal supplement B27 was added to enhance cell survival and preserve microglial function [10,11]. In the subsequent culture phase, macrophage colony-stimulating factor (M-CSF) was supplemented to promote microglial proliferation and expansion [12,13]. This modified protocol not only produced microglia with higher purity but also markedly shortened the isolation timeline, enabling the acquisition of usable primary microglia within as few as 10 days. Moreover, no antibiotic or antifungal agents were used in this protocol. Importantly, the improved method yielded sufficient numbers of more viable cells to support common biochemical analyses—including western blot, polymerase chain reaction (PCR), and proteomic studies—without requiring large numbers of neonatal mice. In addition, the functional competence of the isolated primary microglia was validated through co-culture with lipopolysaccharide (LPS) stimulation (1 μg/mL) [14].
Materials and reagents
Biological materials
1. C57BL/6-Sncatm2.1(SNCA*A53T)Mdk/J (The Jackson Laboratory, strain: #039167)
2. C57BL/6J (The Jackson Laboratory, strain: #000664)
Reagents
1. Phosphate-buffered saline (PBS) 1×, pH 7.2–7.4 (Solarbio, catalog number: P1020)
2. Dulbecco’s modified Eagle medium/nutrient mixture F-12 (DMEM/F-12), 1:1, 1×, supplemented with 2 mM L-glutamine (Gibco, catalog number: 11320033)
3. Trypsin-EDTA solution, 0.25%, 1× (NCM Biotech, catalog number: C100C1)
4. 4% paraformaldehyde fixative solution (Solarbio, catalog number: P1110)
5. B-27TM supplement (50×), serum-free (Thermo Fisher, catalog number: 17504044)
6. Macrophage colony-stimulating factor (M-CSF) (Sigma-Aldrich, catalog number: SRP3221)
7. Fetal bovine serum (FBS), qualified, heat-inactivated (Thermo Fisher, catalog number: 10091148)
Solutions
1. Wash buffer (see Recipes)
2. DMEM/F12 + 10% FBS (see Recipes)
3. DMEM/F12 + 20% FBS (see Recipes)
Recipes
1. Wash buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMED/F12 | n/a | 49 mL |
| B27 (50×) | 1× | 1 mL |
| Total | n/a | 50 mL |
2. DMEM/F12 + 10% FBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMED/F12 | n/a | 45 mL |
| FBS | 2.5 mL/L | 5 mL |
| Total | n/a | 50 mL |
3. DMEM/F12 + 20% FBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMED/F12 | n/a | 40 mL |
| FBS | 5 mL/L | 10 mL |
| Total | n/a | 50 mL |
Laboratory supplies
1. 6-cm cell culture dish (JETBIOFIL, catalog number: TCD010060)
2. 12-well cell culture plate (JETBIOFIL, catalog number: TCP001012)
3. 15-mL centrifuge tube (JETBIOFIL, catalog number: CFT411150)
4. T25 cell culture flask (JETBIOFIL, catalog number: TCF002025)
5. 70-μm cell strainer (JETBIOFIL, catalog number: CSS013070)
6. Glass coverslips (Biosharp, catalog number: BS-18-RC)
Equipment
1. BB15 CO2 incubator (Thermo Scientific, catalog number: 51023126)
2. Multifuge X1 Pro centrifuge (Thermo Scientific, catalog number: 75009750)
3. CS-200 orbital shaker (Yooning, catalog number: CS-200)
4. Dissecting scissors (Fine Science Tools, catalog number: 14160-10)
5. Spring scissors (Fine Science Tools, catalog number: 15018-10)
6. Ophthalmic forceps (Fine Science Tools, catalog number: 11053-10)
7. Dissecting forceps (Fine Science Tools, catalog number: 11000-12)
8. Tissue forceps (Fine Science Tools, catalog number: 11021-12)
9. Scalpel handle and blades (Integra Miltex, catalog number: 4-7)
Procedure
文章信息
稿件历史记录
提交日期: Aug 25, 2025
接收日期: Oct 19, 2025
在线发布日期: Nov 19, 2025
出版日期: Dec 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Li, J., Zheng, Z., Zhang, M., Xue, C., Zhang, X. and Lu, G. (2025). Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction. Bio-protocol 15(23): e5530. DOI: 10.21769/BioProtoc.5530.
分类
神经科学 > 神经系统疾病 > 帕金森氏症
细胞生物学 > 细胞分离和培养 > 细胞分离
神经科学 > 基础技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










