- EN - English
- CN - 中文
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
一种利用自体血浆凝胶生成马源肌性祖细胞的简化三维培养方法
发布: 2025年12月05日第15卷第23期 DOI: 10.21769/BioProtoc.5526 浏览次数: 1259
评审: Olga KopachAnonymous reviewer(s)
Abstract
Musculoskeletal pathologies present challenges in athletic horses, often leading to functional impairment. The slow or limited regenerative capacity of bone, joint, and tendon/ligament injuries, coupled with the limitations of conventional treatments, highlights the need for innovative therapies such as ortho-biologics and mesenchymal stem/stroma cells. Traditional 2D cell culture systems with fetal bovine serum (FBS) fail to replicate the complexity of the in vivo environment, whereas 3D cultures more accurately mimic native tissue architecture and cell–cell interactions. This study describes a novel method for isolating muscle-derived progenitor cells in a 3D environment using an autologous plasma-based gel and an innovative cell retrieval solution. The cultured cells exhibit immunomodulatory effects on T lymphocytes, trilineage differentiation potential, and immunophenotypic characteristics consistent with conventional mesenchymal stem/stromal cells. This streamlined 3D culture technique offers a promising platform for generating minimally manipulated autologous cell products tailored for equine regenerative medicine.
Key features
• Development of a simplified autologous 3D-plasma-based culture method, eliminating the need for fetal bovine serum.
• Fully autologous approach offering safer clinical potential for equine regenerative medicine.
• Easy and time-efficient method suitable for researchers familiar with basic cell culture techniques.
Keywords: 3D cell culture (三维细胞培养)Graphical overview
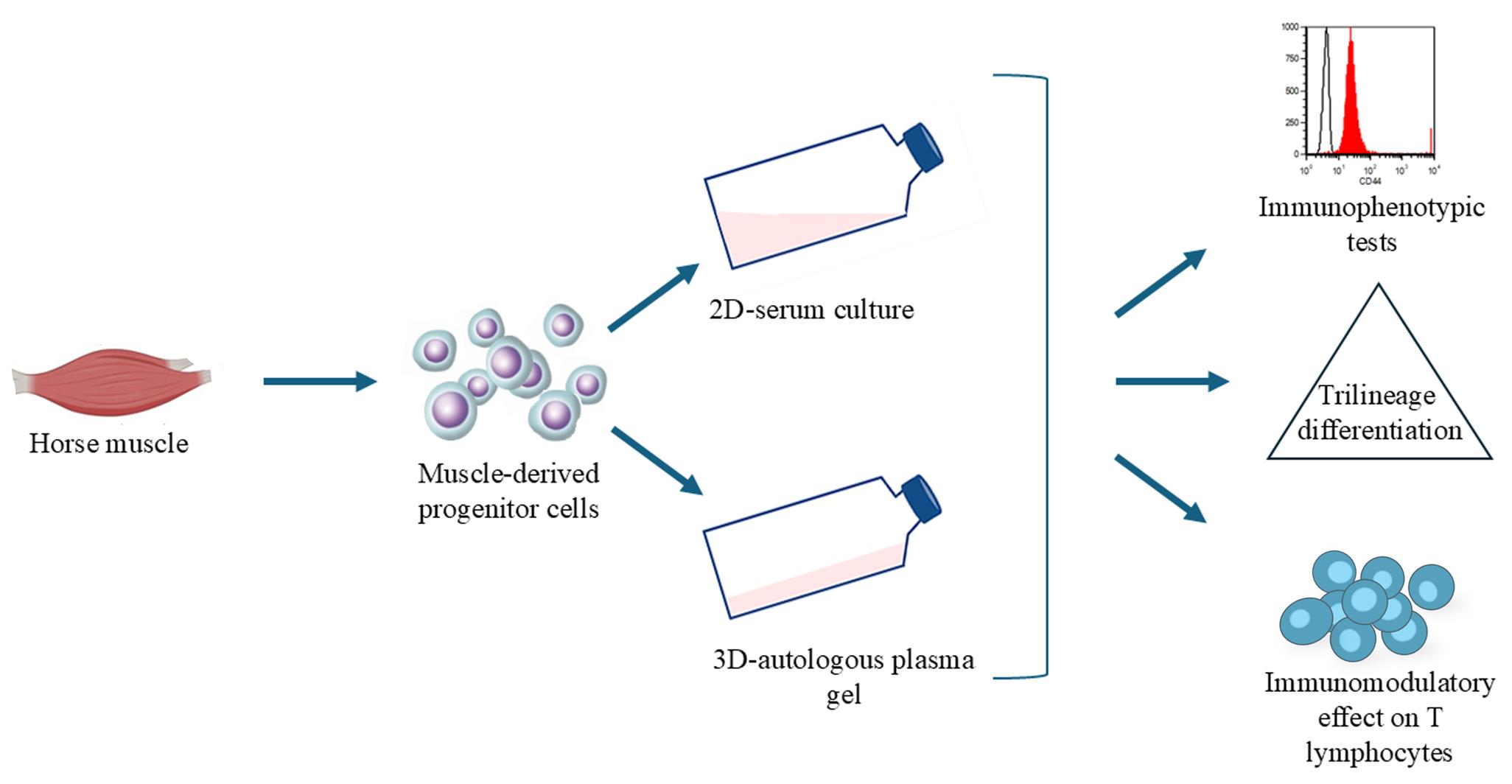
Background
Musculoskeletal disorders are the leading cause of loss of use in athletic horses. In a study of 126 elite show jumpers, 55% of training days lost for medical reasons were due to non-acute orthopedic injuries and 22% to acute injuries [1]. Prevalence increases with age, affecting 51% of horses over 15 years and up to 77% of geriatric horses over 30 [2]. In aged horses, osteoarthritis and chronic laminitis are most common [3–4]. In Thoroughbreds, fracture risk is markedly elevated, prompting research on early warning markers [5]. Standardbreds show lower fracture incidence but higher rates of tendon and suspensory ligament injuries [6].
The limited healing capacity of bone, joint, tendon, and ligament injuries, coupled with high recurrence rates and modest efficacy of conventional therapies, highlights the need for regenerative strategies. Regenerative medicine aims to restore or replace damaged tissues through approaches such as tissue engineering, stimulation of endogenous repair, or mesenchymal stem cell (MSC) therapy [7–8]. In equine practice, several regenerative modalities—platelet-rich plasma (PRP), autologous conditioned serum (ACS), autologous protein solution (APS), and MSCs—are already in clinical use [9–12]. Adult MSCs have been isolated from multiple tissues, with bone marrow and adipose tissue as the main sources. Muscle-derived MSCs (mdMSCs) have also been described across species [13–16]. Ceusters et al. (2017) introduced a simple sampling method using skeletal muscle microbiopsies. Traditionally, mdMSCs are expanded from explants via density-gradient selection and 2D culture in fetal bovine serum (FBS)-supplemented medium, with FBS being an accepted source of growth factors [17].
However, 2D cultures fail to replicate the architecture and physiological conditions of native tissues [18]. In contrast, 3D cultures provide a more biomimetic environment that better reflects tissue architecture, cell–cell interactions, and functional behavior. Advantages include improved modeling of proliferation, differentiation, disease mechanisms, and drug responses, as well as the ability to study complex processes such as angiogenesis and immune interactions. Nevertheless, 3D systems face challenges: technical complexity, variability across protocols, higher costs, and difficulties in imaging and analysis [19–20]. Recently, our group demonstrated the feasibility of expanding mdMSCs in 2D and 3D cultures using 10% equine allogenic platelet lysate as an alternative to FBS [21].
The present study had two main objectives: (i) to establish a novel method for isolating muscle-derived progenitor cells (mdPg cells) in a 3D plasma-based gel (3D plasma) combined with a safe retrieval solution, thereby addressing the need for simplified culture methods to generate minimally manipulated autologous cell products for equine regenerative medicine; and (ii) to evaluate whether cells cultured under these minimally manipulated 3D-plasma conditions retain key mdMSC properties—namely immunophenotype, immunomodulatory activity, and trilineage differentiation capacity—comparable to conventional 2D serum culture.
Materials and reagents
Reagents
1. Phosphate-buffered saline (PBS) without calcium and magnesium (Gibco, catalog number: 14190)
2. Hank’s balanced salt solution (Gibco, catalog number: 24020)
3. TryPLE Express 1× (Gibco, catalog number: 12604)
4. DMEM/Ham’s F12 medium (Gibco, catalog number: 31330)
5. RPMI-1640 medium (Gibco, catalog number: 21875)
6. Amphotericin B (Gibco, catalog number: 15290)
7. Penicillin-streptomycin (Gibco, catalog number: 15140)
8. Fetal bovine serum (FBS) (Gibco, catalog number: A52568)
9. StemPro® Adipognesis Differentiation kit (Gibco, catalog number: A1007001)
10. StemPro® Chondrogenesis Differentiation kit (Gibco, catalog number: A1007101)
11. StemPro® Osteogenesis Differentiation kit (Gibco, catalog number: A1007201)
12. Phytohemagglutinin (Merck, catalog number: 11249738001)
13. Interleukin 2 Human, 15.5 kDa g/mol (Thermo Scientific, catalog number: J67298.EXE)
14. Paraformaldehyde 4% (Merck, catalog number: 1.00496)
15. Stains: trypan blue, alizarin red, oil red O, and alcian blue (Merck, catalog numbers: T8154, TMS-008, 01391, and B8438, respectively)
16. Diff Quick® stain (Alcyon BeLux, catalog number: 10001615)
17. Cell Collect 3D (Revatis, catalog number: R013D)
18. CD44 (CVS18), CD45 (F10-89-4), and MCHII (CVS20) antibodies (Bio-Rad, catalog numbers: MCA1082GA, MCA87, and MCA1085, respectively)
19. CD90 antibodies (Washington State University, catalog number: DH24A)
20. Goat F(ab')2 anti-mouse IgM mu chain (Abcam, catalog number: Ab5926)
21. FACS running buffer (Miltenyi Biotec, catalog number: 130-092-747)
22. CFDA-SE CellTraceTM CFSE Cell Proliferation kit (ThermoScientific, catalog number: V12883)
23. Mepivacaine 20 mg/mL (Intra-Epicaine, GTIN number: 5701170344646)
Solutions
1. DF-12 (see Recipes)
2. DF-20 (see Recipes)
Recipes
1. DF-12
500 mL of DMEM/Ham’s F12 [containing HEPES (15 mM) and glutamine (2.5 mM)] culture medium, with 5 mL of penicillin (1,000 U/mL)-streptomycin (10,000 μg/mL), and 2.5 mL of amphotericin B (250 µg/mL). Total volume: 507.5 mL.
2. DF-20
500 mL of DMEM/Ham’s F12 culture medium with HEPES and glutamine, 100 mL of FBS, 5 mL of penicillin (1,000 U/mL)-streptomycin (10,000 μg/mL), and 2.5 mL of amphotericin B (250 µg/mL). Total volume: 607.5 mL.
It is recommended to use the assembled culture medium within two months.
Laboratory supplies
1. Cell culture equipment, dishes, flasks and blood tubes (Avantor Belgium)
2. Semiautomatic microbiopsy needles 14G (Medicor Belgium, catalog number: MN-701214090)
3. BD Vacutainer 10 mL PET citrate tube (K2EDTA) for haematology (BD Medical, Becton, Dickinson and Company, catalog number: 367525)
Equipment
1. Flow cytometer (Miltenyi Biotec, model: MACSQuant® Analyzer 10)
2. Inverted microscope (Olympus Corporation, model: CKX53)
Software and datasets
1. Cell count and viability assays were performed using the MedCalc Statistical Software (MedCalc Software Ltd., Ostend, Belgium)
Procedure
文章信息
稿件历史记录
提交日期: Aug 28, 2025
接收日期: Oct 22, 2025
在线发布日期: Nov 11, 2025
出版日期: Dec 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Graide, H., Duysens, J., Frank, T., Mouithys-Mickalad, A., Niesten, A., Sandersen, C., Ceusters, J. and Serteyn, D. (2025). A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells. Bio-protocol 15(23): e5526. DOI: 10.21769/BioProtoc.5526.
分类
干细胞 > 成体干细胞 > 肌肉干细胞
细胞生物学 > 细胞分离和培养 > 3D细胞培养
干细胞 > 多能干细胞 > 再生医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












