- EN - English
- CN - 中文
Highly Efficient Agrobacterium-Mediated Transformation of Tomato cv Micro-Tom From Cotyledon Explants
基于子叶外植体的 Micro-Tom 番茄高效根癌农杆菌转化方法
发布: 2025年12月05日第15卷第23期 DOI: 10.21769/BioProtoc.5524 浏览次数: 1500
评审: Noelia ForesiEnzo Ariel PerkAnonymous reviewer(s)
Abstract
The tomato (Solanum lycopersicum) is a widely cultivated crop worldwide that serves as a model system for fruit development studies. Agrobacterium tumefaciens–mediated transformation of tomato has played a central role as a tool for analyzing the function of candidate genes and producing transgenic lines with enhanced resistance to pathogens, tolerance to abiotic stresses, and improved fruit quality traits. Among the many tomato varieties, the miniature dwarf cultivar Micro-Tom (MT) has been increasingly adopted as a model system for tomato research due to its short life cycle, small size, and high transformation efficiency. This protocol outlines a replicable methodology for A. tumefaciens–mediated transformation of Micro-Tom from cotyledon explants, utilizing cost-effective plant growth regulators for shoot regeneration, high transformation rates, reduced regeneration time, and enhanced rooting conditions.
Key features
• Highly efficient tomato genetic transformation from cotyledon explants (up to 80% efficiency rates).
• Fast and reproducible protocol optimized for the Micro-Tom cultivar covering acclimatization steps under greenhouse conditions.
• Utilizes cost-effective plant regulators for shoot regeneration.
Keywords: Plant transformation (植物转化)Background
Tomato (Solanum lycopersicum) is a fleshy fruit crop widely cultivated worldwide, providing a variety of health-promoting compounds for the human diet. Due to its small genome, amenability to stable genetic transformation, and the production of climacteric fleshy fruits, this species is a valuable model system for studies in plant physiology, genetics, and phytopathology, as well as multiple aspects of fruit physiology [1,2]. Tomato cultivars exhibit unique genetic backgrounds, which result in variations in their plant stature and architecture, as well as in fruit size and biochemical composition [3]. Among them, the miniature dwarf Micro-Tom (MT) cultivar offers the advantages of a small size (15–20 cm height), a short life cycle (~3 months), and the ability to grow in high densities [4,5]. The small size of this cultivar is primarily attributed to the dwarf and self-pruning mutations, which restrict brassinosteroid (BR) biosynthesis and result in a determinate growth habit, respectively [3].
Genetic transformation methods are classified as direct (biolistic, electroporation, and PEG-mediated) and indirect (Agrobacterium sp.), based on the mechanism of DNA delivery into plant cells. Among them, the most common methodology is the indirect protocol mediated by Agrobacterium tumefaciens, which utilizes the natural mechanism of this bacterium to transfer a backbone T-DNA into the plant genome. The transformation efficiency via this method is influenced by multiple factors, including the explant source and tissue age, the bacterial strain and concentration, the infection method and duration, and the plant hormones and chemicals used during co-culture and regeneration [6,7].
Tomato transformation is usually performed by using cotyledons of young seedlings as explants [8]. These cotyledons are co-cultivated with Agrobacterium sp. for the insertion of the transgene into the plant; then, by indirect organogenesis, new plants are regenerated and selected [9,10]. Efforts to optimize tomato transformation protocols have led to cumulative increases in transformation rates over the years [4,11,12]. This includes the use of various A. tumefaciens strains, such as LBA4404 [13,14], GH3101 [15], and EHA105 [16,17], with co-cultivation periods ranging from 1 to 3 days. The transformation efficiency reported for Micro-Tom generally depends on explant age, hormone regime, and bacterial strain [13,14,16]. Overall, the transformation rates of tomato typically range from 10% to 65%, depending on the type of explant and Agrobacterium strain [14,16–19]. Most protocols utilize the Murashige and Skoog medium [20] supplemented with zeatin (Z) and indole-3-acetic acid (IAA) at varying concentrations for the shoot regeneration step [21,22]. Additional chemicals, such as acetosyringone, have also been successfully tested to enhance efficiency and reduce the number of required steps [15–17,23–25]. As a critically important tool for both basic and applied research, tomato transformation protocols can significantly benefit from continuous technical refinements. Here, we describe a fast and reproducible Agrobacterium-mediated method optimized for the MT cultivar, with scalability for rapid analysis of gene function, with T0 plants produced in only 4–6 months (Figure 1). This method achieves high efficiency (up to 80%) using cotyledons as explants with the cost-effective replacement of zeatin (Z) for 6-benzylaminopurine (BA) throughout most of the shoot regeneration process. This transformation protocol has been employed to generate stable MT transgenic lines with constitutive and fruit-specific overexpression and RNAi-mediated silencing, as well as CRISPR-mediated knockout mutants, for multiple target genes, allowing the characterization of their functions on vegetative growth, flowering, and fruit development [28–36]. In all cases, homozygous transgenic lines were typically obtained within two or three generations, confirming transgene heritability. Combined with the rapid life cycle of the MT genotype, this relatively fast, straightforward, and highly efficient transformation method, which utilizes less expensive plant hormones, provides a robust system for functional genomics studies in tomato. It enables the analysis of gene function for a larger number of targets more quickly and at a lower cost than alternative protocols.
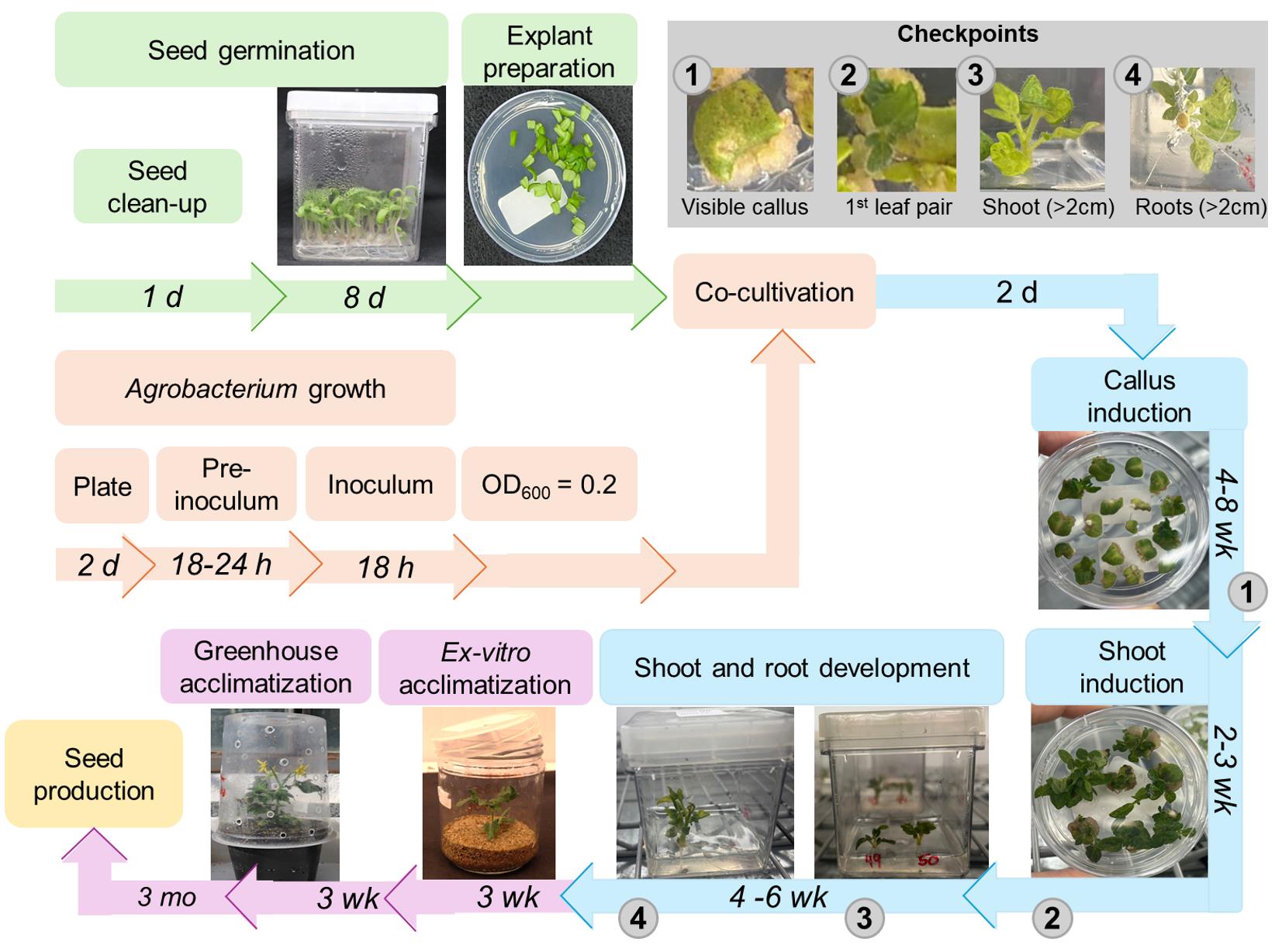
Figure 1. Flowchart for Agrobacterium-mediated transformation and regeneration of tomato (cv. Micro-Tom) using cotyledon explants. Bacterial inoculum preparation and seed germination steps are shown in orange and green, respectively. The shoot/root regeneration and the plant acclimatization steps are indicated in blue and purple, respectively. The estimated time required for each step is shown above the representative images. Gray circles indicate the requisites for transferring the explants to the next stage of the protocol. h: hours, d: days, mo: months, wk: weeks.
Materials and reagents
Biological materials
1. Agrobacterium tumefaciens GV3101
2. Solanum lycopersicum cv Micro-Tom
Reagents
1. (NH4)NO3 (ACS Cientifica, CAS number: R10821000)
2. KNO3 (Sigma-Aldrich, CAS number: S31263-1KG)
3. MgSO4·7H2O (Synth, CAS number: S2035.01.AH)
4. KH2PO4 (Synth, CAS number: F2002.01.AG)
5. CaCl2·2H2O (Synth, CAS number: S1072.01.AH)
6. H3BO3 (Synth, CAS number: A1025.01.AH)
7. MnSO4·7H2O (Synth, CAS number: C2013.01.AH)
8. ZnSO4·7H2O (Synth, CAS number: S1072.01.AH)
9. KI (Sigma-Aldrich, CAS number: 30315-500G)
10. Na2MoO4·2H2O (Synth, CAS number: M1013.01.AE)
11. CuSO4·5H2O (Synth, CAS number: S105.01.AE)
12. CoCl2·6H2O (Synth, CAS number: C1044.01.AE)
13. FeSO4·7H2O (Synth, CAS number: S1057.01.AG)
14. Na2EDTA·2H2O (Sigma-Aldrich, CAS number: E5134-1KG)
15. Nicotinic acid (Synth, CAS number: A1043.01.AG)
16. Pyridoxine-HCl (Sigma-Aldrich, CAS number: P6280-10G)
17. Thiamine-HCl (Sigma-Aldrich, CAS number: T1270-25G)
18. Inositol (Sigma-Aldrich, CAS number: I5125-50G)
19. Sucrose (Synth, CAS number: S2609.01.AM)
20. Phytagel (Sigma-Aldrich, CAS number: P8169-1KG)
21. Agar (HIMEDIA, CAS number: RM301-500G)
22. Acetosyringone (Sigma-Aldrich, CAS number: D134406-5G)
23. 1-Naphthaleneacetic acid (Sigma-Aldrich, CAS number: M0640-25G)
24. Kanamycin (GIBCO, CAS number: 11815)
25. Zeatin (BioBasic, CAS number: 11815-032)
26. Meropenem (ABL Antibióticos do Brasil, CAS number: 1637-39-4)
27. 6-benzylaminopurine (Sigma-Aldrich, CAS number: B3408-5G)
28. Tryptone (HIMEDIA, CAS number: CR014-500G)
29. Yeast extract (KASVI, CAS number: RM301-500G)
30. NaCl (Synth, CAS number: C1060.01.AH)
31. Ethanol P.A (Synth, CAS number: A1083.07.BM)
32. Rifampicin (Sigma-Aldrich, CAS number: 13292-46-1)
33. Gentamycin (Sigma-Aldrich, CAS number: G3632-1G)
34. Polyoxyethylene sorbitan monolaurate (Tween 20) (Sigma-Aldrich, CAS number: P1379)
35. Commercial bleach (2%, v/v)
36. Distilled deionized water
37. HCl (37%) (Synth, CAS number: 13A0079.01.BJ)
38. NPK 10:10:10 (Heringer, CAS number: 66455-26-3)
39. Dolomite limestone (MgCO3 + CaCO3) (Calfertil, CAS number: 1317-65-3)
40. Peters 20:20:20 (Plantafol, Valagro, catalog number: F4)
41. Thermophosphate (Yoorin Master®, Yoorin Fertilizantes, catalog number: I-0009MG)
42. Substrate (Plantmax HT®, Eucatex)
43. Vermiculite (Nutriplan®, catalog number: 8000208)
Solutions
1. Macronutrients (Solution A) (see Recipes)
2. Calcium chloride (Solution B) (see Recipes)
3. Micronutrients (Solution C) (see Recipes)
4. FeEDTA (Solution D) (see Recipes)
5. Vitamins (see Recipes)
6. Acetosyringone (see Recipes)
7. Kanamycin (see Recipes)
8. Meropenem (see Recipes)
9. Naphthaleneacetic acid (NAA) (see Recipes)
10. 6-Benzylaminopurine (BA) (see Recipes)
11. Zeatin (see Recipes)
12. Peter’s solution 5× (see Recipes)
13. Plant media (see Recipes)
a. Germination medium (GM)
b. Virulence induction medium (VIM)
c. Shoot-inducing medium 1 (SIM-1)
d. Shoot-inducing medium 2 (SIM-2)
e. Shoot-inducing medium 3 (SIM-3)
f. Root-inducing medium (RIM)
g. Murashige and Skoog (MS) nutrient solution
h. Yeast extract peptone (YEP) medium
Recipes
1. Macronutrients (Solution A)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| (NH4)NO3 | 33 g/L | 33 g |
| KNO3 | 38 g/L | 38 g |
| MgSO4·7H2O | 7.4 g/L | 7.4 g |
| KH2PO4 | 3.4 g/L | 3.4 g |
| H2O | Up to 1,000 mL |
Keep at 4 °C for up to two months.
2. Calcium chloride (Solution B)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CaCl2·2H2O | 8.8 g/L | 8.8 g |
| H2O | Up to 1,000 mL |
Keep at 4 °C for up to two months.
3. Micronutrients (Solution C)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| H3BO3 | 1.24 g/L | 1.24 g |
| MnSO4·7H2O | 3.38 g/L | 3.38 g |
| ZnSO4·7H2O | 2.12 g/L | 2.12 g |
| KI | 0.166 g/L | 0.166 g |
| Na2MoO4·2H2O | 0.05 g/L | 0.05 g |
| CuSO4·5H2O | 0.005 g/L | 0.005 g |
| CoCl2·6H2O | 0.005 g/L | 0.005 g |
| H2O | Up to 1,000 mL |
Keep at 4 °C for up to two months.
4. FeEDTA (Solution D)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| FeSO4·7H2O | 2.78 g/L | 2.78 g |
| Na2EDTA·2H2O | 3.73 g/L | 3.73 g |
| H2O | Up to 1,000 mL |
Keep at 4 °C for up to two months.
5. Vitamins
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Nicotinic acid | 1 mg/mL | 0.05 g |
| Pyridoxine-HCl | 1 mg/mL | 0.05 g |
| Thiamine-HCl | 5 mg/mL | 0.5 g |
| Inositol | 50 mg/mL | 5 g |
| H2O | Up to 50 mL |
Keep at 4 °C for up to two months.
6. Acetosyringone
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetosyringone | 100 mM | 196 mg |
| Ethanol | Up to 10 mL |
Filter sterilize, aliquot, and store at -20 °C for up to three months.
7. Kanamycin
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Kanamycin | 50 mg/mL | 1.25 g |
| H2O | - | Up to 25 mL |
Filter-sterilize, aliquot, and store at -20 °C for up to three months.
8. Meropenem
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Meropenem | 8.3 mg/mL | 1 g |
| H2O | Up to 120 mL |
Filter-sterilize, aliquot, and store at -20 °C for up to three months. Be aware of the expiration date of the antibiotic.
9. Naphthaleneacetic acid (NAA)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NAA | 0.4 mM | 0.0074 g |
| H2O | Up to 100 mL |
Add 1 M KOH drops to dissolve, then filter-sterilize or autoclave. Aliquot and store at 4 °C for up to six months.
10. 6-Benzylaminopurine (BA)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BA | 0.5 mM | 0.00225 g |
| H2O | Up to 200 mL |
Add some 1 N HCl drops to dissolve, then filter-sterilize, aliquot, and store at -20 °C for up to one year.
11. Zeatin
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Zeatin | 1 mM | 0.0219 g |
| H2O | Up to 100 mL |
Add some 1 N HCl drops to dissolve, then filter-sterilize, aliquot, and store at -20 °C for up to six months.
12. Peter’s solution 5×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Peters 20:20:20 (N:P:K) | 3.5 g/L | 3.5 g |
| H2O | Up to 1,000 mL |
Dilute in tap water and use within one week. Keep at 4 °C.
13. Plant media
a. Germination medium (GM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Solution A | 2.5% (v/v) | 25 mL |
| Solution B | 2.5% (v/v) | 25 mL |
| Solution C | 0.25% (v/v) | 2.5 mL |
| Solution D | 0.5% (v/v) | 5 mL |
| Vitamin | 0.1% (v/v) | 1 mL |
| Sucrose | 1.5% (w/v) | 15 g |
| Phytagel | 0.23% (w/v) | 2.3 g |
| H2O | Up to 1,000 mL |
Adjust pH to 5.8 with KOH and sterilize the media by autoclaving at 121 °C for 15 min and 1.5 atm. In a biosafety cabinet, add approximately 15 mL of the media to each sterilized 15 × 50 mm plate. Keep the media at 4 °C for up to two weeks.
b. Virulence induction medium (VIM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Solution A | 5% (v/v) | 50 mL |
| Solution B | 5% (v/v) | 50 mL |
| Solution C | 0.5% (v/v) | 5 mL |
| Solution D | 1% (v/v) | 10 mL |
| Vitamin | 0.1% (v/v) | 1 mL |
| Sucrose | 3% (w/v) | 30g |
| Agar | 0.6% (w/v) | 6 g |
| Acetosyringone 100 mM | 0.1 mM | 1 mL |
| NAA 0.4 mM | 0.0004 mM | 1 mL |
| H2O | Up to 1,000 mL |
Adjust pH to 5.8 with KOH and sterilize the media by autoclaving at 121 °C for 15 min and 1.5 atm. Then, after partial cooling, add the acetosyringone and NAA. In a biosafety cabinet, add approximately 15 mL of media to each sterilized 15 × 50 mm plate. Keep the media at 4 °C for up to two weeks.
c. Shoot-inducing medium 1 (SIM-1)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Solution A | 5% (v/v) | 50 mL |
| Solution B | 5% (v/v) | 50 mL |
| Solution C | 0.5% (v/v) | 5 mL |
| Solution D | 1% (v/v) | 10 mL |
| Vitamin | 0.1% (v/v) | 1 mL |
| Sucrose | 3% (w/v) | 30 g |
| Agar | 0.6% (w/v) | 6 g |
| Kanamycin 50 mg/mL | 0.05 mg/L | 1 mL |
| Zeatin 0.5 mM | 0.01 mM | 20 mL |
| Meropenem 8.3 mg/mL | 0.03 mg/L | 3.6 mL |
| H2O | Up to 1,000 mL |
Adjust pH to 5.8 with KOH and sterilize the media by autoclaving at 121 °C for 15 min and 1.5 atm. Then, after partial cooling, add the hormones and antibiotics. In a biosafety cabinet, add approximately 15 mL of media to each sterilized 15 × 50 mm plate. Keep the media at 4 °C for up to two weeks.
d. Shoot-inducing medium 2 (SIM-2)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Solution A | 5% (v/v) | 50 mL |
| Solution B | 5% (v/v) | 50 mL |
| Solution C | 0.5% (v/v) | 5 mL |
| Solution D | 1% (v/v) | 10 mL |
| Vitamin | 0.1% (v/v) | 1 mL |
| Sucrose | 3% (w/v) | 30g |
| Agar | 0.6% (w/v) | 6 g |
| Kanamycin 50 mg/mL | 0.1 mg/L | 2.4 mL |
| Zeatin 0.5 mM | 0.003 mM | 6 mL |
| Meropenem 8.3 mg/mL | 0.03 mg/L | 3.6 mL |
| H2O | Up to 1,000 mL |
Adjust pH to 5.8 with KOH and sterilize the media by autoclaving at 121 °C for 15 min and 1.5 atm. Then, after partial cooling, add the phytohormones and antibiotics. In a biosafety cabinet, add approximately 40 mL of media to each sterilized MagentaTM vessel. Keep the media at 4 °C for up to two weeks.
e. Shoot-inducing medium 3 (SIM-3)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Solution A | 5% (v/v) | 50 mL |
| Solution B | 5% (v/v) | 50 mL |
| Solution C | 0.5% (v/v) | 5 mL |
| Solution D | 1% (v/v) | 10 mL |
| Vitamin | 0.1% (v/v) | 1 mL |
| Sucrose | 3% (w/v) | 30 g |
| Agar | 0.6% (w/v) | 6 g |
| Kanamycin 50 mg/mL | 0.1 mg/L | 2.4 mL |
| BA 0.5 mM | 0.005 mM | 10 mL |
| Meropenem 8.3 mg/mL | 0.0075 mg/L | 0.9 mL |
| H2O | Up to 1,000 mL |
Adjust pH to 5.8 with KOH and sterilize the media by autoclaving at 121 °C for 15 min and 1.5 atm. Then, after partial cooling, add the hormones and antibiotics. In a biosafety cabinet, add approximately 40 mL of media to each sterilized MagentaTM vessel. Keep the media at 4 °C for up to two weeks.
f. Root-inducing medium (RIM)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Solution A | 2.5% (v/v) | 25 mL |
| Solution B | 2.5% (v/v) | 25 mL |
| Solution C | 0.25% (v/v) | 2.5 mL |
| Solution D | 0.5% (v/v) | 5 mL |
| Vitamin | 0.1% (v/v) | 1 mL |
| Sucrose | 3% (w/v) | 30 g |
| Phytagel | 0.23% (w/v) | 2.3 g |
| Meropenem 8.3 mg/mL | 0.0075 mg/L | 0.9 mL |
| H2O | Up to 1,000 mL |
Adjust the pH to 5.8 with KOH and sterilize the media by autoclaving at 121 °C for 15 min at 1.5 atm. Then, after partial cooling, add the hormones and antibiotics. In a biosafety cabinet, add approximately 40 mL of the media to each sterilized 15 × 100 mm plate. Keep the media at 4 °C for up to two weeks.
g. Murashige and Skoog (MS) nutrient solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Solution A | 2.5% (v/v) | 25 mL |
| Solution B | 2.5% (v/v) | 25 mL |
| Solution C | 0.25% (v/v) | 2.5 mL |
Solution D H2O | 0.5% (v/v) | 5 mL Up to 1,000 mL |
Adjust the pH to 5.8 with KOH and keep at 4 °C for up to two months.
h. Yeast extract peptone (YEP) medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tryptone | 10 g/L | 10 g |
| Yeast extract | 10 g/L | 10 g |
| NaCl | 5 g/L | 5 g |
| Agar | 9.6 g/L | 15 g |
| H2O | Up to 1,000 mL |
Adjust pH to 7.0 with NaOH and sterilize the media by autoclaving at 121 °C for 15 min and 1.5 atm. In a biosafety cabinet, add approximately 40 mL of the media to each sterilized 15 × 100 mm plate. Keep the media at 4 °C for up to two weeks.
Laboratory supplies
1. Graduated cylinder (100 mL) (Laborglass, catalog number: 9138624)
2. Becker (600 mL) (Laborglass, catalog number: 9110648)
3. Metallic sieve (mesh 100) (ASTM 100, Prolab, catalog number: BEIP022)
4. Metallic spatula (20 cm) (Prolab, catalog number: MER063-1)
5. Plastic petri dish (15 × 100 mm and 15 × 50 mm) (J.Prolab)
6. Fine pointed tweezers (Style AA) (Sigma-Aldrich, catalog number: Z680184)
7. Scissors (Sigma-Aldrich, catalog number: Z265977)
8. Falcon (50 mL) (Corning, catalog number: 430829)
9. Micropipettes (Eppendorf) and tips (Axygen)
10. MagentaTM vessel (Merck, catalog number: V8505, dimension 77 mm × 77 mm × 97 mm)
11. Jars with screw cap for tissue culture (Bio-Sama, catalog number: PP85)
Equipment
1. Centrifuge (ThermoScientific, model: Sorvall ST16R, rotor 75003658–21 cm, 50 mL tube capacity)
2. Bacterial incubator and shaker (Marconi, model: MA832/1)
3. Autoclave (Av plus, Phoenix Luferco)
4. Spectrophotometer (UV 5100 UV/Vis, METASH, Shanghai Inc.)
5. Biosafety cabinets (Esco class II BSC streamline and AMD solutions)
6. Orbital shaking table (TS 2000A VDRL shaker, biomixer)
7. Plant growth chamber (Environplant, Instalafrio)
Procedure
文章信息
稿件历史记录
提交日期: Sep 1, 2025
接收日期: Oct 27, 2025
在线发布日期: Nov 6, 2025
出版日期: Dec 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Pagliuso, D., Rossi, M. and Freschi, L. (2025). Highly Efficient Agrobacterium-Mediated Transformation of Tomato cv Micro-Tom From Cotyledon Explants. Bio-protocol 15(23): e5524. DOI: 10.21769/BioProtoc.5524.
分类
植物科学 > 植物转化 > 农杆菌介导的转化方法
植物科学 > 植物育种 > 微体繁殖
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












