- EN - English
- CN - 中文
A Comparative Protocol for Preserving Deep-Water Marine Invertebrate Tissues: DNA/RNA Shield vs. Liquid Nitrogen for Dual Extraction of High-Quality Nucleic Acids
深海无脊椎动物组织保存的比较方案:DNA/RNA Shield 与液氮在高质量核酸双重提取中的应用对比
发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5521 浏览次数: 1335
评审: Alba BlesaKirsten A. CoprenAnonymous reviewer(s)
Abstract
Preserving biological samples in the field is essential for ensuring high-quality nucleic acid extraction and reliable downstream molecular analyses. Broadly, two main preservation strategies are available: physical preservation, such as flash freezing in liquid nitrogen, which halts enzymatic activity by rapid cooling, and chemical preservation, using stabilizing reagents that inactivate nucleases and protect nucleic acids even at ambient temperatures. This protocol presents a comparative approach using liquid nitrogen and a commercial stabilizing reagent (DNA/RNA Shield, Zymo Research) to preserve tissue from five marine invertebrate species: two cold-water corals, two sponges, and one bivalve. Samples preserved by each method were processed with the AllPrep DNA/RNA Mini kit (Qiagen) to extract both RNA and DNA. RNA quality was assessed using RNA Integrity Number (RIN) scores. The stabilizing reagent preserved high-quality RNA in sponge and bivalve samples but did not prevent RNA degradation in coral tissues, which showed lower RIN scores compared to those preserved in liquid nitrogen. DNA yields were also consistently lower in tissues preserved with DNA/RNA Shield across all species. These findings suggest that DNA/RNA Shield can be a viable alternative to liquid nitrogen for some marine invertebrates, particularly in field conditions where cryopreservation is impractical. However, for cold-water corals, liquid nitrogen remains essential to ensure RNA integrity for transcriptomic analyses and other sensitive molecular applications (e.g., RT-qPCR).
Key features
• Comparative evaluation of preservation methods across five marine invertebrate species.
• Liquid nitrogen is essential for maintaining high RNA integrity in cold-water corals, whereas DNA/RNA Shield effectively preserved RNA in sponges and bivalves.
• Streamlined co-extraction protocol using the AllPrep DNA/RNA Mini Kit (Qiagen) enables reliable recovery of DNA and RNA from field-collected marine samples for downstream molecular applications.
• Integrated quality control workflow (Qubit, Nanodrop, Bioanalyzer RIN) ensuring robust assessment of nucleic acid yield and integrity for downstream molecular applications.
Keywords: RNA preservation (RNA 保存)Graphical overview
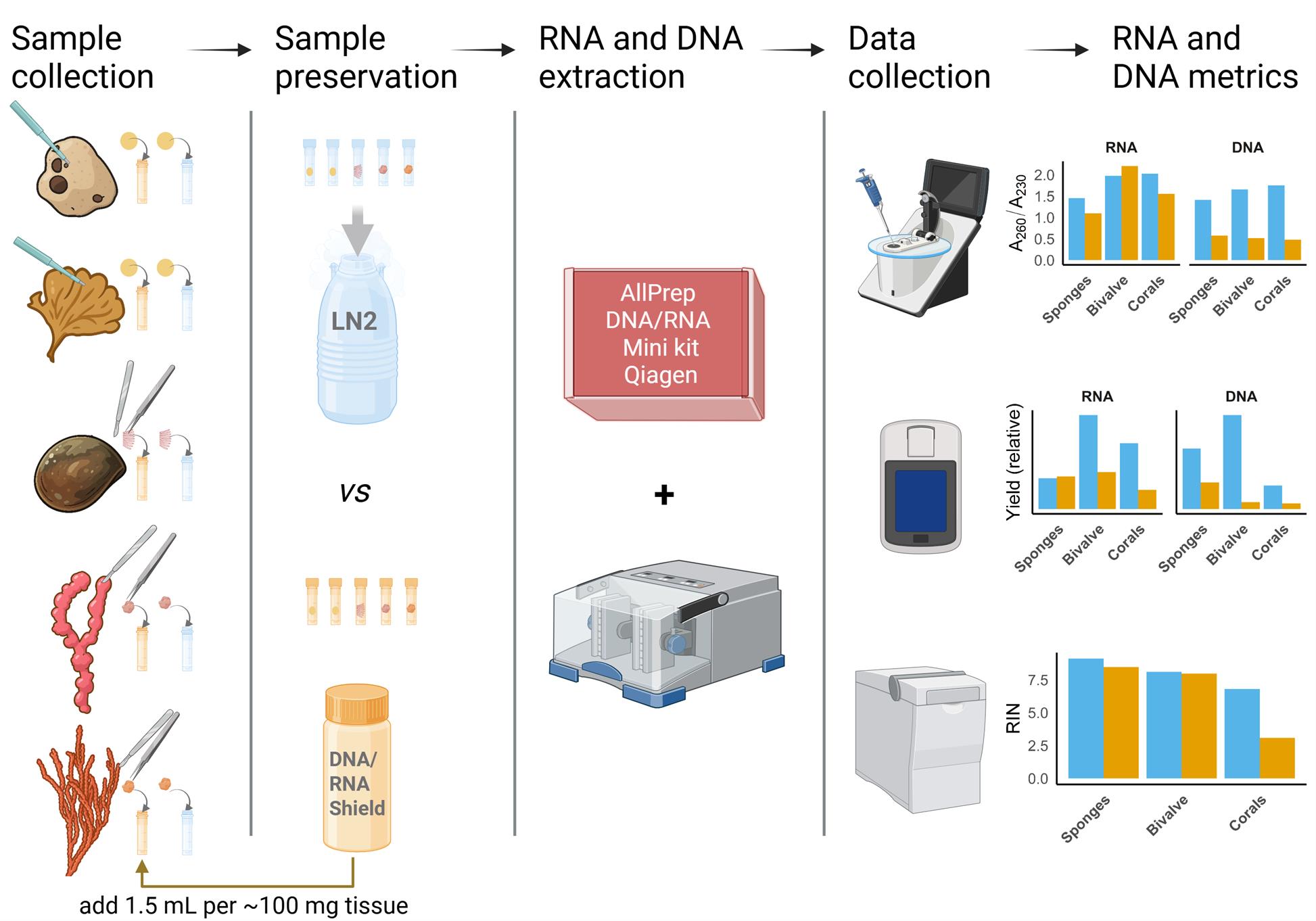
Graphical overview of sample preservation, RNA and DNA co-extraction, and quality assessment protocol for marine invertebrates. Blue indicates samples preserved in liquid nitrogen (LN2); orange indicates samples preserved in DNA/RNA Shield (Zymo Research). Species illustrations were created using ChatGPT based on photos taken by the authors. The graphical abstract was created using https://BioRender.com.
Background
High-quality DNA and RNA are essential for modern molecular applications in ecology, physiology, and environmental monitoring. In marine invertebrate research, particularly involving non-model species such as cold-water corals, sponges, and bivalves, obtaining intact nucleic acids is often complicated by remote sampling locations, tissue heterogeneity, and logistical challenges during field preservation. RNA is particularly unstable and prone to degradation [1], making its preservation a critical step for maintaining its integrity.
Traditionally, flash freezing in liquid nitrogen has been the gold standard for preserving DNA and RNA (see, for example, [2–4]). However, the use of liquid nitrogen is often impractical or hazardous in field settings due to transportation restrictions and the requirement for specialized containers and storage infrastructure. Furthermore, -80 °C storage is frequently unavailable during transit between field sites and centralized research laboratories, and storage or evaporation issues on long trips can further compromise sample integrity. Thus, chemical preservation agents such as DNA/RNA Shield (Zymo Research) offer a more field-adaptable alternative. This reagent stabilizes both DNA and RNA at ambient temperature while simultaneously inactivating nucleases. Prior studies have demonstrated its effectiveness in microbial 16S rRNA gene studies and for preserving RNA in various marine invertebrates [5,6]; however, comparative evaluations of its performance for dual RNA and DNA extraction in diverse marine invertebrates remain limited.
In this study, we evaluated the performance of DNA/RNA Shield in the preservation of nucleic acids relative to liquid nitrogen across five marine invertebrate species, using the AllPrep DNA/RNA Mini kit (Qiagen) for co-extraction. The species are native to the Norwegian coast and include two sponges, Geodia barretti and Phakellia ventilabrum, two soft corals, Paragorgia arborea and Primnoa resedaeformis, and one bivalve, Acesta excavata. These species were selected to represent a diversity of tissue types, structural complexity, and ecological roles. Additionally, all are slow-growing and vulnerable, making them susceptible to human impacts such as bottom trawling and habitat degradation, and therefore important targets for monitoring. RNA integrity and DNA/RNA yield were assessed to identify species-specific preservation outcomes and to determine the feasibility of chemical preservation for multi-taxon molecular sampling. This protocol provides a practical framework for researchers conducting nucleic acid extractions from marine invertebrate tissues in field-limited or remote conditions.
Materials and reagents
Biological materials
Three samples per species (Geodia barretti, Phakellia ventilabrum, Paragorgia arborea, Primnoa resedaeformis, and Acesta excavata) were collected by a remotely operated vehicle (ROV) at a depth range of 115–450 m in the Norwegian coast of Norland and Møre & Romsdal in September 2023 (see Supplementary Information Dataset S1 for sample coordinates and Dataset S2 for examples of species/tissue samples).
Reagents
1. Liquid nitrogen
2. DNA/RNA Shield (Zymo Research, catalog number: R1100–250)
3. Dry ice
4. Commercial bleach (Orkla HPC, Norway)
5. RNaseZap RNase decontamination solution (Invitrogen, catalog number: AM9780)
6. DNA AWAY surface decontaminant (Thermo Scientific, catalog number: 7010)
7. UltraPure DNase/RNase-free distilled water (Invitrogen, catalog number: 10977–035)
8. 14.3 M 2-mercaptoethanol (Merck, catalog number: M6250–250ML)
9. 96% Ethanol (VWR, catalog number: 20824.365)
10. AllPrep DNA/RNA Mini kit (Qiagen, catalog number: 80204)
11. Qubit dsDNA Quantification BR (Invitrogen, catalog number: Q32850)
12. Qubit RNA Quantification BR (Invitrogen, catalog number: Q10210)
13. Bioanalyzer RNA 6000 Nano assay (Agilent Technologies, catalog number: 5067-1511)
Solutions
1. 10% bleach solution (see Recipes)
2. 50% bleach solution (see Recipes)
3. 70% ethanol (see Recipes)
Recipes
1. 10% bleach solution
To prepare 200 mL of 10% bleach, add 20 mL of commercial bleach solution to 180 mL of distilled water.
2. 50% bleach solution
To prepare 50 mL of 50% bleach in a 50 mL Falcon tube, mix 25 mL of commercial bleach solution with 25 mL of distilled water. The solution should be prepared fresh daily, and more frequently (2–3 times per day) if needed.
3. 70% ethanol
To prepare 50 mL of 70% ethanol in a 50 mL Falcon tube, mix 36 mL of 96% ethanol with 14 mL of UltraPure DNase/RNase-free distilled water.
Laboratory supplies
1. Disposable gloves
2. Unicore punch kit 6.00 mm (Merck, catalog number: WHAWB100082)
3. Scalpel blades No. 11 (Swann-Morton, catalog number: 0303)
4. CryoPure tubes 2 mL (Sarstedt, catalog number: 72.379)
5. Stainless steel beads 5 mm (Qiagen, catalog number: 69989)
6. Screw cap micro tubes 2 mL sterile (Sarstedt, catalog number: 72.694.005)
7. Weighing boats, sterile (VWR, catalog number: 611-5272)
8. 10 μL pipette tips (VWR, catalog number: 732-3631)
9. 100 μL pipette tips (VWR, catalog number: 732-3633)
10. 200 μL pipette tips (VWR, catalog number: 732-3634)
11. 1,000 μL pipette tips (VWR, catalog number: 732-3637)
12. Collection tubes 2 mL (Qiagen, catalog number: 19201)
13. Qubit assay tubes (Invitrogen, catalog number: Q32856)
14. Lens paper (Hecht Glaswarenfabrik GmbH & Co KG, catalog number: 41019010)
15. PCR tubes 0.2 mL (VWR, catalog number: 732-0548)
16. Falcon 50 mL conical centrifuge tubes (Fisher Scientific, catalog number: 10203001)
17. Falcon 15 mL conical centrifuge tubes (Fisher Scientific, catalog number: 11507411)
Equipment
1. Cryogenic GT26 Dewar (Air Liquide, catalog number: GT26-1)
2. Forceps (Fine Science Tools, catalog number: 00632-11)
3. Scalpel handle No. 3 (Fine Science Tools, catalog number: 10003-12)
4. Plastic cutting boards (standard kitchen cutting boards)
5. Centrifuges (Fisher scientific accuSpi Micro 17 Microcentrifuge, catalog number: 13-100-675 and Eppendorf MiniSpin plus, catalog number: 5453000015)
6. Fume hood (Labflex, model: 625)
7. Ice machine (Migel ice line, model: KF85)
8. TissueLyser II homogenizer (Qiagen, catalog number: 85300)*
9. TissueLyser Adapter Set 2 × 24 (Qiagen, catalog number: 69982)
10. Nanodrop One spectrophotometer (Thermo Fisher, catalog number: ND-ONE-W)
11. Qubit 2.0 fluorometer (Invitrogen, catalog number: Q32866)
12. Vortex-Genie2 (Scientific Industries, Inc., model: SI-0256)
13. Prime Thermal Cycler (Bibby Scientific, catalog number: 3PRIMEX/02)
14. Agilent 2100 Bioanalyzer (Agilent, catalog number: G2938C)*
15. Chip Priming Station (Agilent, catalog number: 5065-4401)
16. MS2 S8 minishaker (IKA Works Inc.)*
17. Freezer able to maintain -80 °C
18. Fridge able to maintain 4 °C
19. Micropipette P2, Eppendorf Research plus (Eppendorf, catalog number: 3123000012)
20. Micropipette P10, Eppendorf Research Plus (Eppendorf, catalog number: 3123000020)
21. Micropipette P20, Eppendorf Research Plus (Eppendorf, catalog number: 3123000039)
22. Micropipette P100, Eppendorf Research Plus (Eppendorf, catalog number: 3123000047)
23. Micropipette P200, Eppendorf Research Plus (Eppendorf, catalog number: 3123000055)
24. Micropipette P1000, Eppendorf Research Plus (Eppendorf, catalog number: 3123000063)
25. Wash bottle 500 mL (VWR, catalog number: 215-0111)
Note: * indicates equipment that has been discontinued by the manufacturer.
Software and datasets
1. Microsoft Office Excel (Microsoft Office 365), or similar
2. R version 4.3 or later [7]
3. R packages: tidyverse [8], emmeans [9], ggplot2 [10], readxl [11], janitor [12], cowplot [13]
4. Agilent Software (for RIN scoring)
Procedure
文章信息
稿件历史记录
提交日期: Aug 12, 2025
接收日期: Oct 14, 2025
在线发布日期: Oct 31, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Gomes, A. S., Guerreiro, E. M., Pochon, X., Keeley, N. and Laroche, O. (2025). A Comparative Protocol for Preserving Deep-Water Marine Invertebrate Tissues: DNA/RNA Shield vs. Liquid Nitrogen for Dual Extraction of High-Quality Nucleic Acids. Bio-protocol 15(22): e5521. DOI: 10.21769/BioProtoc.5521.
分类
环境生物学
分子生物学 > RNA > RNA 提取
分子生物学 > DNA > DNA 提取
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












