- EN - English
- CN - 中文
Quantifying Intestinal Glucose Absorption Using Isolated Vascularly Perfused Rat Small Intestine
利用离体灌流大鼠小肠模型定量分析肠道葡萄糖吸收
发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5514 浏览次数: 1584
评审: Wendy Leanne HempstockAnonymous reviewer(s)
Abstract
Intestinal glucose absorption has been studied for several decades. However, the different methods available for investigating absorption are often the reason for variability in the results, and it is difficult to measure the relative contribution of paracellular absorption using existing methods. Thus, we have established a new model for measuring glucose absorption. In the isolated in situ vascularly perfused small intestine, the intestinal epithelium is completely preserved, and the entire transport pathway is intact. In the present model, we use radioactive labeled 14C-d-glucose, which allows for sensitive quantification of glucose absorption even with low luminal concentrations. The described method is optimized for intestinal glucose absorption but can be applied to other macro/micronutrients that can be radioactively labeled. The described procedure is a novel approach for measurements of intestinal nutrient absorption and gut permeability in which luminal nutrient concentrations resemble physiological concentrations.
Key features
• Sensitive quantification of intestinal glucose absorption at physiologically relevant luminal glucose concentrations.
• Accurate distinction between transcellular vs. paracellular glucose absorption using mannitol as an indicator of paracellular intestinal absorption.
• Differentiation between apical and basolateral pathways for transport across the intestinal epithelium.
Keywords: Nutrient absorption (营养吸收)Graphical overview
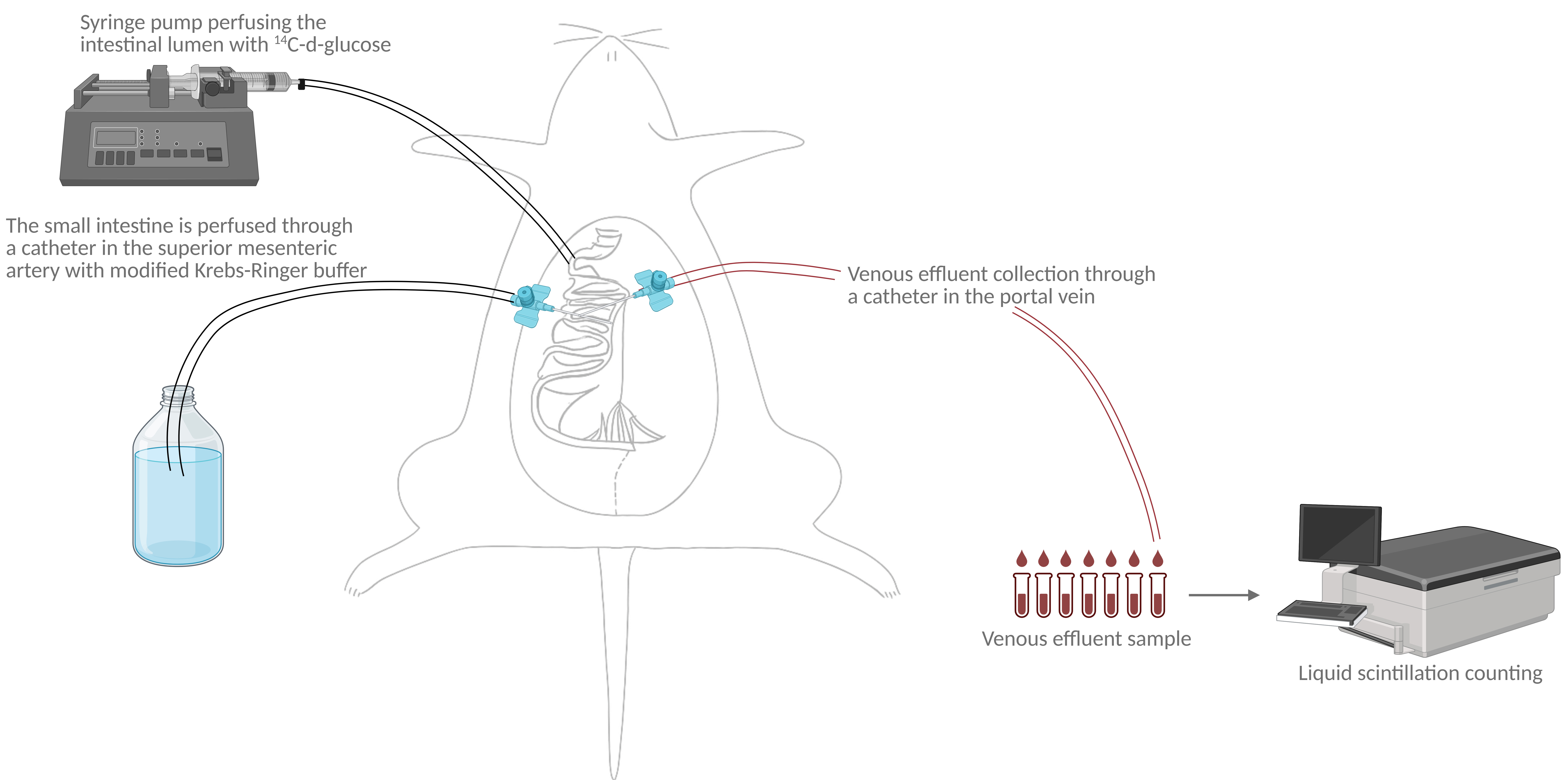
Illustration of perfusion setup and analysis. The graphical overview illustrates the study setup. The rat's small intestine is surgically isolated. The small intestine is vascularly perfused through a catheter in the superior mesenteric artery while stimulating the intestinal lumen with 14C-d-glucose. Venous effluent samples are collected in 1-min intervals through a catheter in the portal vein. Radioactivity (14C) in venous effluent samples is subsequently measured using liquid scintillation counting.
Background
Glucose absorption has been studied extensively since the 1960s, when Crane et al. [1] described a transepithelial glucose transporter later named sodium-glucose transporter 1 (SGLT1). Subsequently, in the 1980s, the low-affinity glucose transporter 2 (GLUT2) was characterized [2]. Since then, both transcellular (using SGLT1 and GLUT2) and paracellular glucose absorption have been studied using several in vivo, ex vivo, and in vitro methods [3–7]. A widely used in vivo method is the oral glucose tolerance test (OGTT) [8] with stable or radioactive tracers (e.g., [13C]- or [3H]-glucose) [9,10] to track absorption kinetics. While being physiologically relevant, the method is not intestine-specific, neither is it possible to determine the relative contributions of transcellular and paracellular absorption. Another widely used method is the ex vivo method involving the Ussing chamber [11,12], in which segments of intestinal mucosa are mounted between two half-chambers to separately bathe/perfuse mucosal and serosal sides and subsequently measure glucose flux. This method does not allow differentiating between transcellular and paracellular absorption; further, the method is limited to small tissue samples and is inadequate regarding transport kinetics. In vitro cell culture models, such as the Caco-2 monolayers (a colon-derived cell line), are beneficial for mechanistic and molecular studies of transepithelial transport; however, they lack physiological context, as cell culture models do not represent the complexity of the intestinal epithelium and do not allow measurements of adequate flow rates [13–15].
In rodent in vivo models, single-pass intestinal perfusion glucose absorption has been measured in a defined intestinal segment. Previously, glucose absorption was measured by applying a controlled concentration of glucose to the intestinal lumen and then collecting the luminal outflow to measure unabsorbed glucose [16–18]. However, the sensitivity of that method may be a problem, as measuring this small difference requires highly accurate glucose assays, and small assay errors may mask real absorption. In the present method, we employ a single-pass perfusion system, in which the small intestine is surgically isolated and vascularly perfused via the superior mesenteric artery with a modified Krebs-Ringer buffer. The intestinal lumen is stimulated with glucose labeled with a radioactive tracer [14C-d-glucose]; this radioactive tracer is subsequently measured in the venous effluent drawn from the portal vein. With this model, the cytoarchitecture and whole transport pathway is completely preserved, mimicking in vivo conditions. Further, is it possible to surgically isolate a specific intestinal segment, allowing comparison between different gut regions [19,20].
Materials and reagents
Animals
Male Wistar rats (~250 g, 8–10 weeks old) were purchased from Janvier (Saint Berthevin Cedex, France) based on weight. It is possible to use both smaller and larger rats; however, using both may make it difficult to perform some critical steps of the surgery [e.g., placing the arterial catheter in smaller rats (<200 g); for larger rats (>400 g), the greater amount of visceral fat may prolong the surgery]. Rats are housed in pairs in an air-conditioned (21 °C) and humidity-controlled (55%) room and kept in a 12/12 h light/dark cycle with ad libitum access to standard chow and drinking water. Rats are not fasted before the surgery. Female rats may be used; however, one needs to be aware of the effects of the female reproductive system.
Reagents
1. Sodium chloride (NaCl) (Sigma-Aldrich, CAS: 7647-14-5)
2. Calcium chloride dihydrate (CaCl2·2H2O) (Merck, catalog number: 102382)
3. Magnesium sulfate heptahydrate (MgSO4·7H2O) (Merck, CAS: 10034-99-8)
4. Potassium dihydrogen phosphate (KH2PO4) (Merck, catalog number: 105886)
5. Sodium bicarbonate (NaHCO3) (Merck, CAS: 144-55-8)
6. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: 1.12018.0500)
7. Dextran T-70 (Pharmacosmos, CAS: 9004-54-0)
8. Fumaric acid (Merck, catalog number: 76635)
9. Glutamate (Merck, catalog number: 106445)
10. Pyruvate (Merck, catalog number: 106619)
11. D-glucose (Sigma-Aldrich, catalog number: G8270)
12. Glucose, d-[1-14C] (Revity, catalog number: NEC043X)
13. Ultima Gold (Revity, catalog number: 6013321)
14. Potassium chloride (KCl) (Merck, CAS: 7447-40-7)
15. Sodium hydroxide (NaOH) (Merck, CAS: 1710-73-2)
16. Hydrogen chloride (HCl) (Merck, CAS: 7647-01-0)
17. D-mannitol (Sigma-Aldrich, catalog number: M4125)
18. Mannitol, d-[2-14C] (Revity, catalog number: NEC852050UC)
19. Ketamine (VetViva, catalog number: QN01AX03)
20. Dexmedetomidine (Orion Pharma, catalog number: QN05CM18)
Solutions
1. Isotonic saline (see Recipes)
2. Modified Krebs-Ringer buffer (see Recipes)
3. Glucose solution (see Recipes)
4. Mannitol solution (see Recipes)
Recipes
1. Isotonic saline
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | 0.9% w/v | 9.0 g |
| MilliQ | 1,000 mL |
Isotonic saline should be sterile. Prepare the saline in advance. It can be stored at 4 °C.
2. Modified Krebs-Ringer buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BSA | 0.1% w/v | 1.0 g |
| Dextran T-70 | 5% w/v | 50.0 g |
| D-glucose | 3.5 mmol/L | 0.631 g |
| Fumaric acid | 5 mmol/L | 0.800 g |
| Glutamate | 5 mmol/L | 0.936 g |
| Pyruvate | 5 mmol/L | 0.550 g |
| NaCl | 118 mmol/L | 6.9 g |
| KCl | 4.7 mmol/L | 0.35 g |
| CaCl2·2H2O | 2.5 mmol/L | 0.37 g |
| MgSO4·7H2O | 1.2 mmol/L | 0.30 g |
| KH2PO4 | 1.2 mmol/L | 0.16 g |
| NaHCO3 | 25 mmol/L | 2.10 g |
| MilliQ | 1,000 mL |
The buffer can be made in advance and stored at -20 °C in aliquots of 1 L. Move the needed amount from the freezer to 4 °C and thaw overnight the day before the perfusion protocol.
3. Glucose solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| D-glucose | 100 mmol/L | 0.360 g |
| Isotonic saline | 20 mL | |
| Glucose, d-[1-14C] | 20 μL |
4. Mannitol solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| D-mannitol | 100 mmol/L | 0.360 g |
| Isotonic saline | 20 mL | |
| Mannitol, d-[2-14C] | 20 μL |
Laboratory supplies
1. MINISORP, Nunc-immuno tube (Thermo Fisher, catalog number: 468608)
2. Q-tips, medical cotton swaps (Bio-Medical, catalog number: MDS202000)
3. Coors Buchner Funnel (Merck, catalog number: Z247340)
4. Qualitative filter paper (Merck, catalog number: WHA1001110)
Equipment
Surgical tools
1. Surgical scissors, straight, blunt (Anima Lab, catalog number: 14000-12FST)
2. Surgical fine scissors, straight (Anima Lab, catalog number: 14060-12FST)
3. Graefe forceps, curved (Anima Lab, catalog number: 11051-10FST)
4. Vannas spring scissors (microscissors) (Anima Lab, catalog number: 1508-10FST)
5. Qtips, medical cotton swaps (Bio-Medical, catalog number: MDS202000)
6. Black sterile silk 5-0 (Vömel, catalog number: 14739)
7. Absorptive compression gauze 5 × 5 cm (Mölnlycke Health Care, catalog number: 156015)
8. 10 mL plastic syringe for luminal stimuli (Merck, catalog number: Z683590)
Single-pass perfusion system (Hugo Electronics-Harvard apparatus, March-Hugstetten, Germany)
9. Universal perfusion system (Harvard Bioscience Inc., catalog number: 73-2316)
10. Basic Unit Uniper UP-100, Type 834 (Harvard Bioscience Inc)
11. Roller Pump (Harvard Bioscience Inc., catalog number: 75-1000)
12. Windkessel (Harvard Bioscience Inc., catalog number: 73-2068)
13. Thermostatic Circulator Bath (Harvard Bioscience Inc., catalog number: 73-2802)
14. Operating table with tripod (Harvard Bioscience Inc, catalog number: PY2 73-3776)
15. Cannula with basket and side port, OD = 1.3 mm, ID = 1.0 mm (Harvard Bioscience Inc, catalog number: 73-3311)
16. Cannula with basket, OD = 2.0 mm, ID = 1.5 mm (Harvard Bioscience Inc, catalog number: 73-3312)
17. Plastic tube for luminal stimulus (Merck, catalog number: BR143255-20M)
18. Coors Buchner Funnel (Merck, catalog number: Z247340)
19. Qualitative filter paper (Merck, catalog number: WHA1001110)
20. Benchmark magnetic stirrer (Merck, catalog number: Z742551)
21. Magnetic stir bar (Merck, catalog number: HS120551)
22. Fraction collector, F9-R (Äkta, catalog number: 29011362)
Glucose measurements
23. Quantulus GCT 6220 liquid scintillation counter (Revity, catalog number: A622000)
24. 6 mL pony vial for liquid scintillation counting (Revity, catalog number: 6000292)
Procedure
文章信息
稿件历史记录
提交日期: Aug 27, 2025
接收日期: Oct 14, 2025
在线发布日期: Nov 2, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bæch-Laursen, C., Bæch-Laursen, S. and Holst, J. J. (2025). Quantifying Intestinal Glucose Absorption Using Isolated Vascularly Perfused Rat Small Intestine. Bio-protocol 15(22): e5514. DOI: 10.21769/BioProtoc.5514.
分类
生物化学 > 糖类 > 葡萄糖
细胞生物学 > 组织分析 > 生理学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












