- EN - English
- CN - 中文
Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
(§Technical contact: Ilyssa.Ramos@nih.gov) 发布: 2025年11月20日第15卷第22期 DOI: 10.21769/BioProtoc.5512 浏览次数: 2351
评审: Durai SellegounderAnonymous reviewer(s)

相关实验方案

基于人外周血单个核细胞(PBMCs)和浆细胞样树突细胞(pDCs)的宿主靶向抗病毒药物(HTA)筛选方案
Zhao Xuan Low [...] Pouya Hassandarvish
2025年03月05日 3068 阅读
Abstract
Protein phosphorylation is a dynamic post-translational modification that regulates fundamental processes, including signal transduction, cell proliferation, differentiation, and effector function of immune cells. The Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway is a key mediator of cytokine responses, essential for maintaining immune cell homeostasis and determining cell fate across diverse immune subsets. Dysregulation of JAK/STAT signaling has been linked to a broad spectrum of pathologies, including monogenic immune disorders, autoimmunity, and cancer. Platforms facilitating single-cell analysis of protein phosphorylation offer the ability to reveal subtle signaling defects and dissect the pleiotropy in cellular composition and phosphorylation status, providing insights into immune phenotype and function, while identifying potential therapeutic targets. While an application of cytometry-by-time-of-flight, termed phospho-CyTOF, has proven invaluable for studying protein phosphorylation in cryopreserved peripheral blood mononuclear cells (cPBMCs), its application is limited by cell loss and signaling artifacts stemming from isolation and cryopreservation. Conversely, whole blood (WB) approaches, preserving the native immune cell composition and signaling context, offer a more physiological representation but necessitate robust and consistent protocols for broad application. Herein, we present optimized dual phospho-CyTOF workflows tailored for both cPBMCs and whole blood, building upon established protocols for cytokine stimulation of both samples. These workflows facilitate comprehensive, high-dimensional profiling of JAK/STAT signaling in response to pleiotropic cytokines such as Type I interferons (IFN-α), Type II interferons (IFN-γ), and Interleukin-21 (IL-21). By leveraging CyTOF's capacity for high-dimensional profiling using pure heavy metal–labeled antibodies, these protocols aim to identify pathway-specific alterations in STAT phosphorylation across major immune subsets that may be overlooked by traditional flow cytometry. Together, these optimized dual workflows provide scalable, translationally relevant tools for dissecting the subtle and differential JAK/STAT-driven immune responses in both clinical and research settings, while also being compatible with the simultaneous assessment of crosstalk with alternative immune cell signaling pathways.
Key features
• This method enables multiplexed detection of 20 surface markers and STAT phosphorylation to resolve subsets and interrogate diverse JAK/STAT signaling.
• Whole blood workflow supports rapid “vein-to-tube” processing and fixation, preserving native signaling, immune cell composition, and fragile myeloid subsets.
• Designed for users with CyTOF expertise who are proficient in cytometry workflows involving surface and intracellular staining, fixation, and phospho-epitope preservation across immune subsets.
• Applicable to clinical immunomonitoring, pharmacodynamic studies (i.e., JAK inhibitors), and biomarker discovery in immune dysregulation.
Keywords: Mass cytometry (质谱流式细胞术)Graphical overview
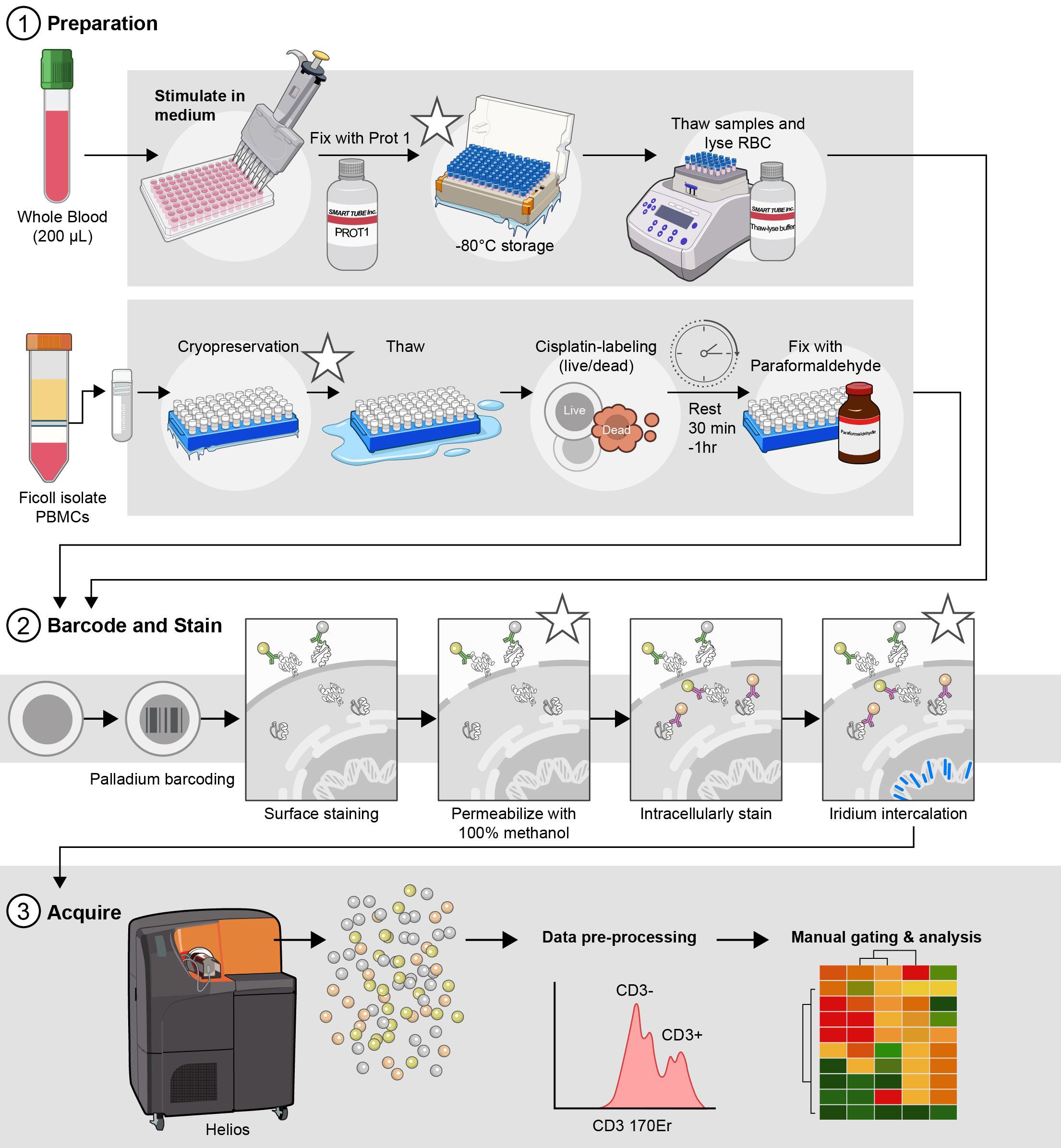
Optimized parallel workflows for assessing immune cell signaling via Phospho-CyTOF. (1) Sample preparation and stimulation: cryopreserved peripheral blood mononuclear cells (cPBMCs) or whole blood are stimulated with IFN-α, IFN-γ, or IL-21 to induce specific JAK/STAT phosphorylation events. Immediately following stimulation, cells are fixed to preserve phosphorylation states: cPBMCs with 2.2% paraformaldehyde (PFA) and WB samples with Prot1 fixative (Smart Tube, Inc.). Whole blood samples then undergo erythrocyte lysis using thaw/lyse solution (Smart Tube, Inc.). (2) Barcoding, staining, permeabilization, intracellular staining, and iridium intercalation: Both cPBMC and lysed WB samples are subsequently palladium-barcoded for multiplexing, followed by surface staining to identify major immune cell subsets. To facilitate intracellular staining, cells are permeabilized overnight in 100% methanol. The following day, samples are rehydrated and stained for intracellular phospho-proteins (e.g., pSTAT1, pSTAT3, pSTAT4, pSTAT5, and pSTAT6) and other intracellular markers (e.g., pP38, pERK1/2, IKβα). To exclude doublets during acquisition, cells are incubated in iridium intercalation solution. (3) Sample acquisition: Prior to acquisition, samples are resuspended at an optimized concentration to achieve approximately 350 events per second (± 50 events per second) and diluted 1:10 with EQ Four Element Calibration Beads (Standard BioTools) in cell acquisition solution plus (CAS+). These data are acquired with a Helios mass cytometer. *Indicates potential stopping points in the protocol.
Background
Protein phosphorylation is a reversible post-translational modification that plays a central role in regulating immune cell signaling, activation, differentiation, and effector functions [1]. This process is orchestrated by kinases and phosphatases, which add or remove phosphate groups from serine, threonine, or tyrosine residues on intracellular signaling proteins [2]. Dysregulation of these signaling events is linked to the pathogenesis of various diseases, including autoimmune disorders, autoinflammatory syndromes, immunodeficiencies, allergies, and malignancies.
Intracellular signal transduction is a crucial cell communication process that transfers extracellular signals regulating activation, survival, proliferation, and differentiation in immune cells [3]. Pathway activation is regulated by the binding of cytokines, inflammatory mediators, hormones, and extracellular messengers to cell surface receptors, which initialize signal transducers (i.e., kinases and transcription factors) [4] to drive cell phenotype and function. The strict temporal and spatial regulation of activated signal transducers drive major cellular decisions, such as cell cycle checkpoints, apoptosis, and cytoskeletal reorganization [5]. Studying changes in cellular communication methods (i.e., phosphorylation) under various conditions can provide a deeper understanding of underlying biological processes, emphasizing the importance of understanding how these signaling pathways are stimulated and regulated [6].
The Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway is a crucial signaling cascade that orchestrates a diverse array of cellular processes essential for immune homeostasis and host defense. Due to its pleiotropy, this pathway mediates the effects of multiple cytokines (>50), interferons, and growth factors that dictate cell fate, proliferation, migration, metabolism, differentiation, and effector function across all immune subsets [7] (Figure 1). In mammals, the JAK family comprises four proteins, JAK1-3 and TYK2, whereas the STAT family contains seven proteins (STAT1-4, STAT5A/5B, and STAT6). Even within the same family, different cytokines engage unique JAK-STAT combinations, resulting in distinct transcriptional programs that modulate cellular responses [8]. For example, Type I interferons (IFN-α), Type II interferons (IFN-γ), and Interleukin-21 (IL-21) are potent immune modulators that typically activate diverse, sometimes overlapping, STAT molecules to drive biological outcomes, from antiviral and anti-tumor immunity to T follicular helper cell differentiation and B-cell responses. Mounting evidence elucidates the role of dysregulated JAK/STAT signaling in inborn errors of immunity (IEI) and cancer, underscoring the need to effectively interrogate this pathway in complex biological samples.
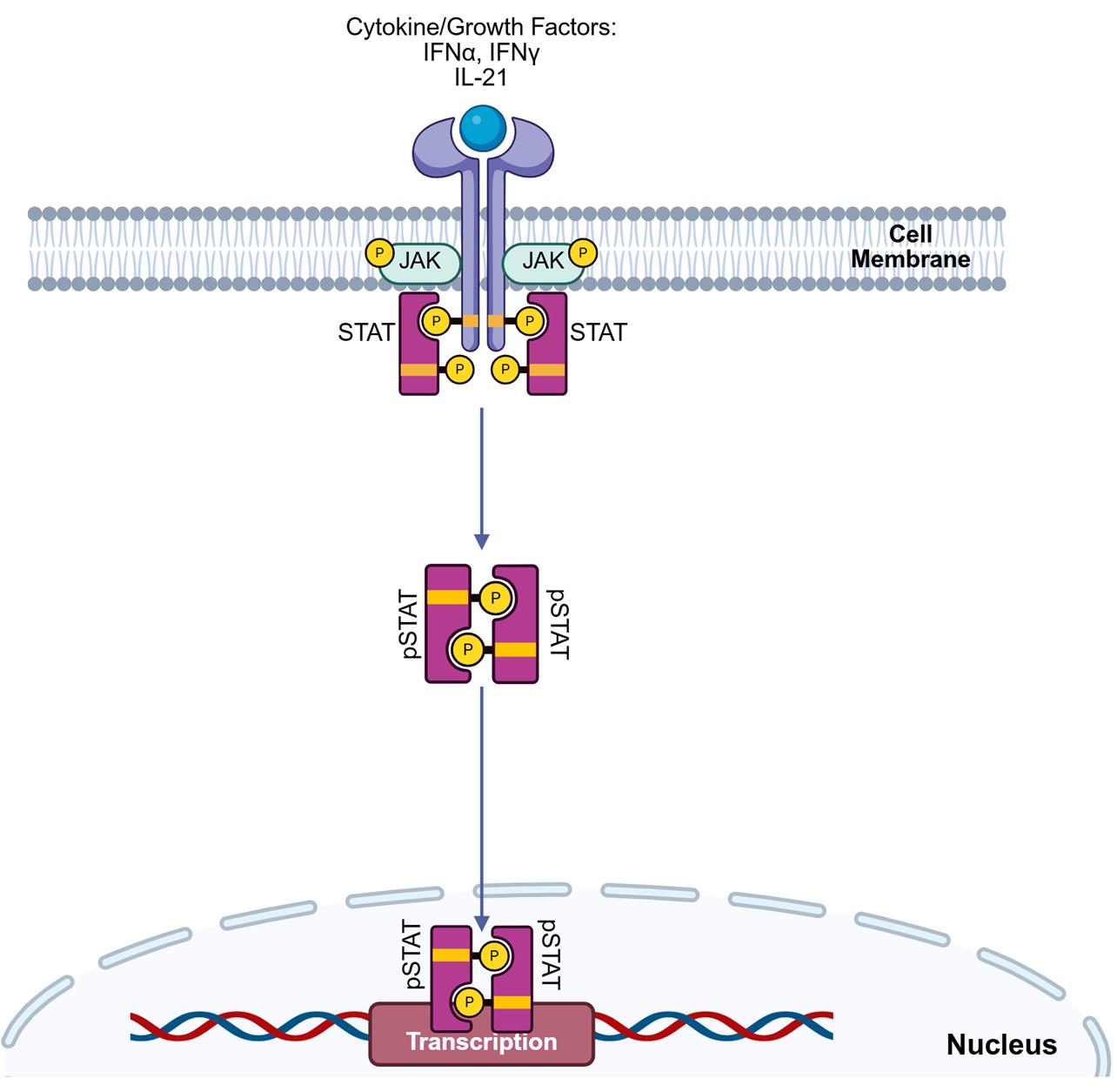
Figure 1. Extracellular receptor engagement triggers intracellular STAT phosphorylation in immune cells. The binding of extracellular cytokines, growth factors, or interferons to their cognate receptors initiates a rapid intracellular signaling program. This phenomenon triggers autophosphorylation of Janus-associated kinases (JAK), followed by sequential docking and phosphorylation/activation of signal transducer and activator of transcription (STAT) proteins. Phosphorylated STATs dimerize and translocate into the nucleus, driving transcriptional programs that lead to pro- or anti-inflammatory phenotypes in immune cells.
A thorough assessment of JAK/STAT signaling is critical for understanding immune dysregulation in disease and in guiding the development and deployment of targeted therapies. Traditional methods for studying protein phosphorylation, such as western blot and ELISA, have limitations due to their bulk nature, low throughput, and inability to resolve signaling at the single-cell level [8]. Fluorescence-based phospho-flow cytometry enables multiplexed, single-cell analysis; however, it faces constraints such as spectral overlap, compensation issues, and autofluorescence [9]. Mass cytometry, also known as cytometry by time-of-flight (CyTOF), has emerged as a state-of-the-art technology that utilizes heavy metal–conjugated antibodies for the detection of proteins, thereby circumventing the limitations of flow cytometry and enabling simultaneous measurements of over 40 parameters per cell, including both surface markers and intracellular phospho-epitopes [10,11]. This high-dimensional method, phospho-CyTOF, is a powerful tool for the simultaneous assessment of STAT phosphorylation across key immune cell subsets with single-cell resolution.
In translational immunology, a significant challenge is the choice of the biological sample matrix, i.e., cryopreserved peripheral blood mononuclear cells (cPBMCs) vs. whole blood (WB). Despite the invaluable advantage of longitudinal sample banking and batch processing, the isolation of PBMCs and cryopreservation can induce cellular stress, alter basal phosphorylation states, and potentially impact cellular responsiveness [12]. Conversely, whole blood stimulation offers a more physiologically relevant "vein-to-tube" approach, maintaining a full cellular repertoire that preserves cell-to-cell interactions that occur in vivo, despite presenting logistical hurdles for large-scale, multi-center studies.
To address this fundamental question, we present optimized dual phospho-CyTOF workflows that can be used to investigate the pleiotropic effect of cytokine stimulation on JAK/STAT signaling in immune cells from both cryopreserved PBMCs [13,14] and whole blood [15]. These protocols enable concurrent immunophenotyping and phospho-protein profiling that is compatible with high-dimensional data analysis pipelines. To ensure translational relevance, we adapted a protocol introduced in both Fernandez and Maecker’s 2015 Bio-Protocols for cPBMCs [13,14] and whole blood [15]. Specifically, we improve upon cell loss that is experienced following repeated fixation and the methanol permeabilization steps, implement palladium barcoding to limit batch variability in staining, and expand the intracellular panel to include six of seven STAT molecules for more comprehensive functional profiling. The overarching goal is to directly compare and refine a protocol for each sample type, thereby aiding researchers in leveraging the strengths and weaknesses of both approaches to address their specific biological question. Furthermore, by mapping differential phosphorylation of STAT1, STAT3, STAT4, STAT5, and STAT6 in response to the cytokines IFN-α, IFN-γ, and IL-21, across major immune subsets, this protocol provides invaluable insights into the nuanced and context-dependent nature of JAK/STAT signaling in human immunity for immediate clinical application. Understanding differential phospho-STAT responses across sample types is crucial for designing robust immunomonitoring studies and for precisely interpreting results in diverse research and clinical settings that may contribute to understanding human immunity and driving therapeutic strategies in instances of dysregulation.
Materials and reagents
Biological materials
1. Human whole blood preserved in sodium heparin tubes (NIH Clinical Center Blood Bank)
2. Cryopreserved peripheral blood mononuclear cells (cPBMCs) (NIH Clinical Center Blood Bank)
Reagents
1. IFNα (PBL, catalog number: 11105-1)
2. IFNγ (BD Biosciences, catalog number: 554617)
3. Lipopolysaccharide (LPS) from Salmonella enterica serotype enteritidis γ-irradiated, BioXtra, suitable for cell culture (Sigma-Aldrich, catalog number: L7770-1MG)
4. IL-21 (Fisher Scientific, catalog number: PHC0214)
5. RPMI 1640 medium, GlutaMAXTM supplement (Thermo Fisher Scientific, catalog number: 61870036)
6. Heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific, catalog number: A5670801)
7. Dulbecco’s modified PBS without Ca2+/Mg2+ (Thermo Fisher Scientific, catalog number: 14190094)
8. Penicillin-streptomycin (10,000 U/mL) (pen/strep) (Thermo Fisher Scientific, catalog number: 15140122)
9. 16% formaldehyde solution (w/v) methanol-free (Thermo Fisher Scientific, catalog number: 28906)
10. Cell-ID Cisplatin-195Pt (Standard BioTools, catalog number: 201195)
11. Pierce Universal nuclease for cell lysis, 25 kU (nuclease inhibitor) (Thermo Fisher Scientific, catalog number: 88701)
12. Prot1 (SmartTube Inc., catalog number: 501351691)
13. Thaw/lyse concentrate (SmartTube Inc., catalog number: 501351696)
14. Methanol (MeOH) ACS reagent, ≥99.8%, 1 L (Sigma-Aldrich, catalog number: 179337-1L)
15. Water, molecular biology (Quality Biological, catalog number: 351-029-101)
16. Maxpar 10× barcode perm concentrate (50 mL) (Standard BioTools, catalog number: 201057)
17. Maxpar PBS (500 mL) (Standard BioTools, catalog number: 201058)
18. Maxpar® cell staining buffer (500 mL) (Standard BioTools, catalog number: 201068)
19. Cell-IDTM 20-Plex Pd Barcoding kit (Standard BioTools, catalog number: 201060)
20. Cell-IDTM Intercalator-Ir, 12.5 μM (500 μL) (Standard BioTools, catalog number: 201192C)
21. EQ Four Element calibration beads (100 mL) (Standard BioTools, catalog number: 201078)
22. Maxpar® fix and perm buffer (100 mL) (Standard BioTools, catalog number: 201067)
23. Maxpar® cell acquisition solution plus for CyTOF® XT (1,000 mL) (CAS+) (Standard BioTools, catalog number: 201244)
24. Maxpar® water (500 mL) (Standard BioTools, catalog number: 201069)
25. CyTOF tuning solution (250 mL) (Standard BioTools, catalog number: 201072)
Solutions
1. Thawing media (see Recipes)
2. Cisplatin labeling media (see Recipes)
3. 2× Cell-ID Cisplatin-195Pt working solution (see Recipes)
4. Stimulation media (see Recipes)
5. 1× thaw/lyse buffer (see Recipes)
6. 1× barcoding buffer (BC buffer) (see Recipes)
7. Cell-IDTM Intercalator-Ir Solution (iridium intercalation solution) (see Recipes)
8. 1:10 EQ Four Element calibration beads (1:10 EQ4 beads) (see Recipes)
Recipes
1. Thawing media
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 medium, GlutaMAXTM supplement | 89% | 44.495 mL |
| Heat-inactivated FBS | 10% | 5.000 mL |
| Penicillin-streptomycin (10,000 U/mL) | 1% | 0.500 mL |
| Pierce Universal nuclease for cell lysis | 10,000× | 0.005 mL |
| Total | 50.000 mL |
a. Alternatively, aliquots of stimulation media (Recipe 4) can be used as a base to directly add Pierce Universal nuclease for cell lysis.
b. Prepare thawing media immediately before use and discard remaining media once thawing is complete.
c. Warm the media in a 37 °C water bath to thaw the PBMCs.
2. Cisplatin labeling media
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 medium, GlutaMAXTM supplement | 99% | 495 mL |
| Penicillin-streptomycin (10,000 U/mL) | 1% | 5 mL |
| Total | 500 mL |
a. Filter-sterilize the cisplatin labeling media through a 500 mL filtration unit with a 0.22 μm pore size.
b. Media can be stored at 4 °C for up to one month.
c. Warm the cisplatin labeling media in a 37 °C water bath to label PBMCs.
3. 2× Cell-ID Cisplatin 195Pt working solution
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 medium, GlutaMAXTM supplement | 9.995 mL | |
| Cell-ID Cisplatin-195Pt | 2,000× | 0.005 mL |
| Total | 10.0 mL |
a. Prepare the 2× Cell-ID Cisplatin-195Pt working solution immediately before use and discard the remaining solution once labeling is complete.
b. Add 0.5 μL/mL of 2× Cell-ID Cisplatin-195Pt working solution to the appropriate volume of cisplatin labeling media to create a 2× Cell-ID Cisplatin-195Pt working solution.
4. Stimulation media
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 medium, GlutaMAXTM supplement | 89% | 445 mL |
| Heat-inactivated fetal bovine serum (FBS) | 10% | 50 mL |
| Penicillin-streptomycin (10,000 U/mL) | 1% | 5 mL |
| Total | 500 mL |
Filter-sterilize the stimulation media through a 500 mL 0.22 μm filtration unit. Stimulation media can be prepared up to one month before use and stored at 4 °C.
5. 1× Thaw/lyse buffer
| Reagent | Final concentration | Volume |
|---|---|---|
| Water, molecular biology | NA | 49.95 mL |
| Thaw/lyse concentrate (1,000×) | 1× | 0.05 mL |
| Total | 50 mL |
a. Filter-sterilize the 1× thaw/lyse buffer through a 500 mL 0.22 μm filtration unit.
b. 1× thaw/lyse buffer can be prepared and stored at room temperature (RT) for up to one month from the preparation date.
6. 1× Barcoding buffer (BC buffer)
| Reagent | Final concentration | Volume |
|---|---|---|
| MaxPar PBS | NA | 45 mL |
| Maxpar 10× barcode perm concentrate | 1× | 5 mL |
| Total | 50 mL |
1× BC buffer can be stored at 4 °C and used up to one month from the preparation date.
7. Iridium intercalation solution
| Reagent | Final concentration | Volume |
|---|---|---|
| Maxpar® fix and perm buffer | NA | 3.998 mL |
| Cell-IDTM Intercalator-Ir, 12.5 μm | 2,000× | 0.002 mL |
| Total | 4.000 mL |
a. The iridium intercalation solution should be prepared immediately before use and stored on ice.
b. Iridium intercalator stock should be aliquoted immediately after thawing.
c. After a second thaw, aliquots of iridium intercalator stock should be discarded.
8. 1:10 EQ4 beads
| Reagent | Final concentration | Volume |
|---|---|---|
| Maxpar® cell acquisition solution plus for CyTOF® XT (CAS+) | NA | 9 mL |
| EQ Four Element calibration beads | 10× | 1 mL |
| Total | 10 mL |
a. The EQ Four Element calibration beads require vigorous shaking by hand for at least 30 s prior to use.
b. Do not vortex the EQ Four Element calibration beads, as it fails to resuspend the beads into solution.
c. The 1:10 EQ Four Element calibration beads in CAS+ (1:10 EQ4 Beads) should be prepared immediately before use and stored on ice.
Laboratory supplies
1. 25 mL sterile reservoirs (Thermo Fisher Scientific, catalog number: 95128095)
2. DNA LoBind tube 2.0 mL (Eppendorf, catalog number: 022431048)
3. Safe-Lock tubes 1.5 mL, natural (Eppendorf, catalog number: 022363212)
4. Dry Ice
5. Matrix tube 0.5 mL 2D screw tubes PP, V-bottom, w/clear caps (Thermo Fisher Scientific, catalog number: 3744)
6. Matrix tube 0.5 mL 2D screw tubes PP, V-bottom, w/blue caps (Thermo Fisher Scientific, catalog number: 3744BLU)
7. Matrix tube 0.5 mL 2D screw tubes PP, V-bottom, w/pink caps (Thermo Fisher Scientific, catalog number: 3744PIN)
8. Matrix tube 0.5 mL 2D screw tubes PP, V-bottom, w/green caps (Thermo Fisher Scientific, catalog number: 3744GRE)
9. Matrix tube 0.5 mL 2D screw tubes PP, V-bottom, w/yellow caps (Thermo Fisher Scientific, catalog number: 3744YEL)
10. Millipore® Stericup® quick release vacuum filtration system (Millipore Sigma, catalog number: S2GPU10RE)
11. 14 mL polystyrene round-bottom tube (Corning, catalog number: 352051)
12. 5 mL polystyrene round-bottom tube with cell strainer cap (Falcon, catalog number: 352235)
13. 50 mL polypropylene conical tube 30 × 115 mm style (Corning, catalog number: 352070)
14. RTS LTS 1,200 μL filter 768/4 tips (Rainin, catalog number: 17007084)
15. RTS LTS 1,000 μL filter 768/4 tips (Rainin, catalog number: 17014967)
16. RTS LTS 200 μL F 960A/10 (Rainin, catalog number: 30389239)
17. Dualfilter 200 μL, PCR clean/sterile (Eppendorf, catalog number: 022491296)
18. P20 LTS pipette tips (Rainin, catalog number: 17014392)
19. Sterile 5 mL serological pipette (VWR Scientific, catalog number: 612-3702)
20. Sterile 10 mL serological pipette (VWR Scientific, catalog number: 89130-898)
21. Plate seal (VWR Scientific, catalog number: 734-4000)
22. 96-well deep well 2 mL plate (Fisher brand, catalog number: 12-566-121)
23. 96-well V-bottom plate with lid, polystyrene, TC-treated (Corning, catalog number: 3894)
24. 96-well U-bottom plate with lid, polystyrene, TC-treated (Corning, catalog number: 3799)
25. C-Chip (inCyto, catalog number: DHC-N01-5)
Equipment
1. Helios CyTOF (Standard BioTools, model: PN 400250 A7)
2. Cellaca MX high-throughput cell counter (Revvity, catalog number: MX-AOPI)
3. Thermo Sorvall Legend XTR refrigerated centrifuge (Marshall Scientific, catalog number: TSO-LEGXTR)
4. CorningTM mini microcentrifuge, 100–240V (Fisher Scientific, catalog number: 07-203-954)
5. -86 °C upright freezer (Phcbi, catalog number: MDFDU702VHPA)
6. -20 °C K2 scientific laboratory undercounter freezer (Fisher Scientific, catalog number: K204SDF)
7. 4 °C TSX Series high-performance lab refrigerator (Thermo Fisher Scientific, catalog number: TSX3005SD)
8. EppendorfTM Centrifuge 5430 R, refrigerated microcentrifuge (Fisher Scientific, catalog number: 13-690-005)
9. Water bath (Corning, catalog number: 6783)
10. Pipet-Lite Pipette, Universal SL-1000XLS+ (Mettler Toledo, catalog number: 17014407)
11. Pipet-Lite Pipette, Universal SL-200XLS+ (Mettler Toledo, catalog number: 17014391)
12. Pipet-Lite LTS Pipette L-20XLS+ (Mettler Toledo, catalog number: 17014392)
13. Pipet-Lite LTS Pipette L-10XLS+ (Mettler Toledo, catalog number: 17014388)
14. Pipet-Lite LTS Pipette L-2XLS+ (Mettler Toledo, catalog number: 17014393)
15. Pipet-Lite Pipette Multi L12-1200XLS+ (Mettler Toledo, catalog number: 17014497)
16. Pipet-Lite Multi Pipette L12-300XLS+ (Mettler Toledo, catalog number: 17013811)
17. Pipet-Lite Multi Pipette L12-200XLS+ (Mettler Toledo, catalog number: 17013810)
18. CorningTM 8-channel adapter for vacuum aspirator (Corning, catalog number: 4931)
19. Pipet-Aid® XP (Drummond Scientific Company, catalog number: 4-000-101)
20. Eppendorf® Thermomixer® FP (Millipore Sigma, catalog number: EP5385000024)
21. Vortex Genie-2 (Millipore Sigma, catalog number: Z258423)
22. FormaTM Series II water-jacketed CO2 incubator (Thermo Fisher, catalog number: 3131)
23. 1300 Series Class II, Type A2 Biological Safety Cabinet Packages (Thermo Fisher, catalog number: 1323TS)
24. Microscope (Olympus, catalog number: CKX53)
25. Milli Q® IQ 7000 ultrapure water purification system (Millipore Sigma, catalog number: ZIQ7000T0C)
Software and datasets
1. Fluidigm Software (Standard BioTools, Version 7.1), license required, https://www.fluidigm.com/software
2. Excel (Microsoft, Version 2408), license required, https://www.microsoft.com/en-us/microsoft-365/excel
3. FlowJo (TreeStar, Version 10.10), license required, https://www.flowjo.com/solutions/flowjo/downloads/
4. GraphPad Prism (GraphPad Software LLC., Version 9.5.0), license required, https://www.graphpad.com/scientific-software/prism/
5. RStudio (Posit Software, PBC, Version 2024.09.1+394), no license required, https://posit.co/download/rstudio-desktop/
Procedure
文章信息
稿件历史记录
提交日期: Aug 2, 2025
接收日期: Oct 12, 2025
在线发布日期: Oct 30, 2025
出版日期: Nov 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Ramos, I. E., Matthiasardottir, B., Hawley, T. S., Han, K. L., Toborek, M., Douagi, I., Jones, G. N. and Cherry, J. M. (2025). Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood. Bio-protocol 15(22): e5512. DOI: 10.21769/BioProtoc.5512.
分类
免疫学 > 免疫细胞功能 > 细胞因子
细胞生物学 > 细胞信号传导 > 胞内信号传导
分子生物学 > 蛋白质 > 磷酸化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









