- EN - English
- CN - 中文
Lipid-Mediated Sequential Recruitment of Proteins Via Dual SLIPT and Dual SLIPTNVOC in Live Cells
基于双SLIPT与双SLIPT-NVOC的脂质介导蛋白依序定位活细胞研究方法
(§Technical contact: kristina.bayer@mr.mpg.de) 发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5489 浏览次数: 1603
评审: Abhilash PadavannilAnonymous reviewer(s)
Abstract
Cellular phenomena such as signal integration and transmission are based on the correct spatiotemporal organization of biomolecules within the cell. Therefore, the targeted manipulation of such processes requires tools that can precisely induce the localizations and interactions of the key players relevant to these processes with high temporal resolution. Chemically induced dimerization (CID) techniques offer a powerful means to manipulate protein function with high temporal resolution and subcellular specificity, enabling direct control over cellular behavior. Here, we present the detailed synthesis and application of dual SLIPT and dual SLIPTNVOC, which expand the SLIPT (self-localizing ligand-induced protein translocation) platform by incorporating a dual-ligand CID system. Dual SLIPT and dual SLIPTNVOC independently sort into the inner leaflet of the plasma membrane via a lipid-like anchoring motif, where they present the two headgroup moieties trimethoprim (TMP) and HaloTag ligand (HTL), which recruit and dimerize any two iK6eDHFR- and HOB-tagged proteins of interest (POIs). Dual-SLIPTNVOC furthermore enables this protein dimerization of POIs at the inner leaflet of the plasma membrane in a pre-determined order and light-controlled manner. In this protocol, we detail the synthetic strategy to access dual SLIPT and dual SLIPTNVOC, while also providing the underlying rationale for key design and synthetic decisions, with the aim of offering a streamlined, accessible, and broadly implementable methodology. In addition to the detailed synthesis, we present representative applications and typical experimental outcomes and recommend strategies for data analysis to support effective use of the system. Notably, dual SLIPT and dual SLIPTNVOC represent the first CID systems to emulate endogenous lipidation-driven membrane targeting, while retaining hallmark advantages of CID technology—the precision over POI identity, recruitment sequence, high spatiotemporal control, and “plug-and-play” flexibility.
Key features
• Expands the original SLIPT technology [1] by enabling plasma membrane (PM) recruitment of any two POIs and their dimerization.
• Dual SLIPTNVOC as the first self-localizing lipid-like probe to induce PM recruitment and dimerization, with a defined recruitment sequence.
• Optimal use case at low probe concentrations: the system mimics physiological lipid-mediated dimerization without globally saturating the plasma membrane with recruited POIs.
• Descriptions of solid phase peptide synthesis and chemical synthesis for facile access to dual SLIPT and dual SLIPTNVOC, experimental results, and their analysis
Keywords: SLIPT technology (SLIPT技术)Graphical overview
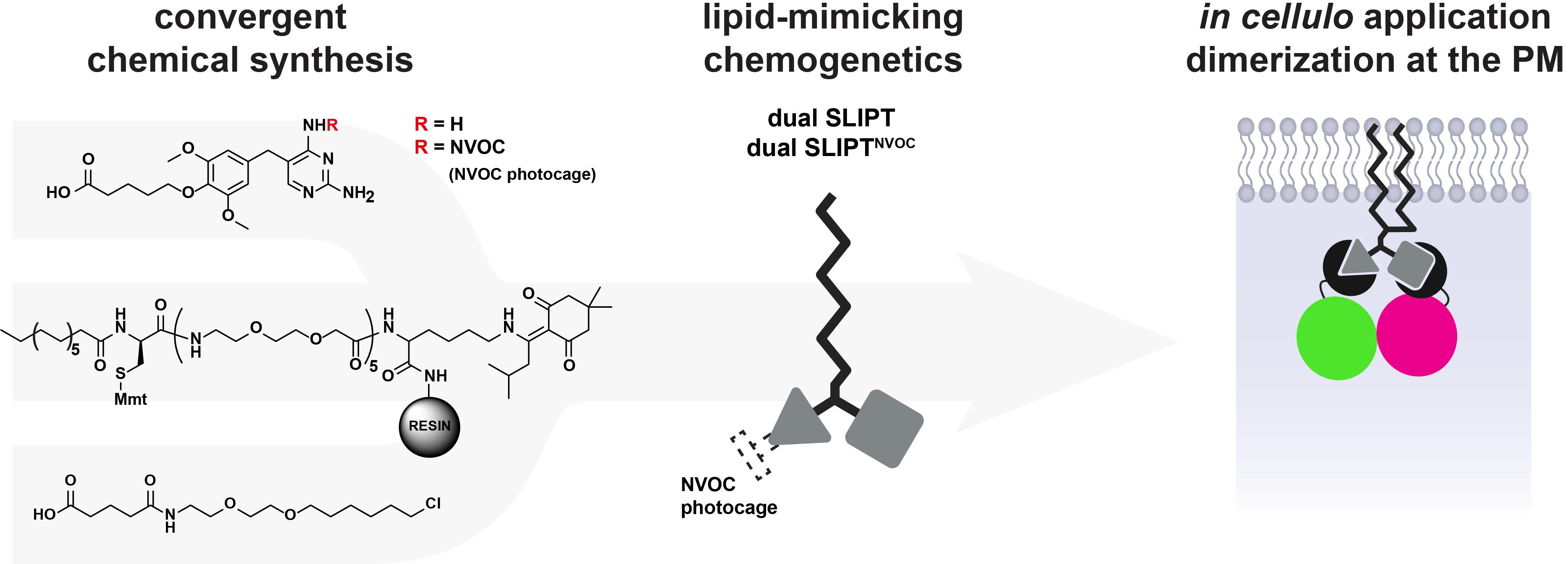
From chemical synthesis to in cellulo dimerization
Background
Spatiotemporal control of the localization and interactions of proteins of interest (POIs) using light as an external trigger is a highly attractive and minimally invasive manipulation strategy. Optogenetic or chemogenetic tools have emerged as a pivotal technique for establishing cause-and-effect and synthetically programming cellular behavior. The protocol presented herein details the synthesis and application of dual SLIPT and dual SLIPTNVOC [2], which expand on the self-localizing ligand-induced protein translocation (SLIPT) technology [1] with a chemically inducible dimerization (CID) approach.
Dual SLIPT and dual SLIPTNVOC comprise a (opto-)chemical tool set that enables recruitment of any two POIs to the inner leaflet of the plasma membrane (PM) (Figure 1). Dual SLIPT independently sorts into the PM via a lipid-like localization motif and recruits the two POIs by presenting two headgroup moieties [Trimethoprim (TMP) and HaloTag ligand (HTL)], which are bound by the two protein-tags iK6eDHFR (eDHFR variant [3]) and Halo-based oligonucleotide binder (HOB [4]). Thus, dual SLIPT makes use of a minimal number of mutually orthogonal protein tags to translocate two POIs in parallel from the cytosol to the entire inner leaflet of the PM and induce dimerization upon addition of the probe to the cell.
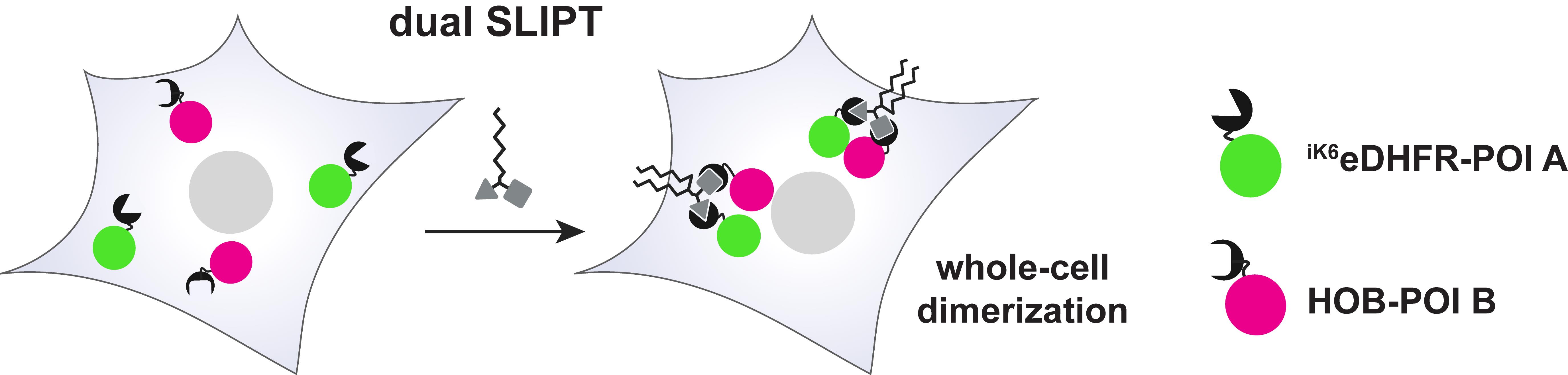
Figure 1. Mode of action of dual SLIPT. Cells expressing iK6eDHFR-POI A and HOB-POI B are treated with dual SLIPT. This causes dual recruitment of both fusion proteins to the inner leaflet of the plasma membrane inside the entire cell (whole-cell dimerization), which induces the dimerization of POI A and POI B.
Dual SLIPTNVOC, on the other hand, presents one constitutively active headgroup (HTL) and one conditionally active (photocaged TMPNVOC) headgroup to the cytosol. Upon addition to the cell, HOB-tagged POIs are immediately recruited to the inner leaflet of the PM, whereas TMPNVOC remains inactive with respect to PM recruitment of iK6eDHFR-tagged POIs (Figure 2). Irradiation with blue light allows for photouncaging the TMP headgroup, rendering it active and causing the co-recruitment of iK6eDHFR-tagged POIs within seconds [2]. Thus, dual SLIPTNVOC offers light-controlled, sequential activation for greater spatiotemporal precision.
Use of dual SLIPT and dual SLIPTNVOC has been demonstrated in identifying sequence-dependent effects of protein localization at the plasma membrane, as occurs during signal integration, propagation, and crosstalk, by controlling lamellipodia via sequential PM recruitment of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) [2].
Furthermore, by using dual SLIPTNVOC, the precise control over the light-irradiated area enables spatially confined dimerization to specific subcellular regions, thereby closely emulating endogenous lipid-mediated protein recruitment.
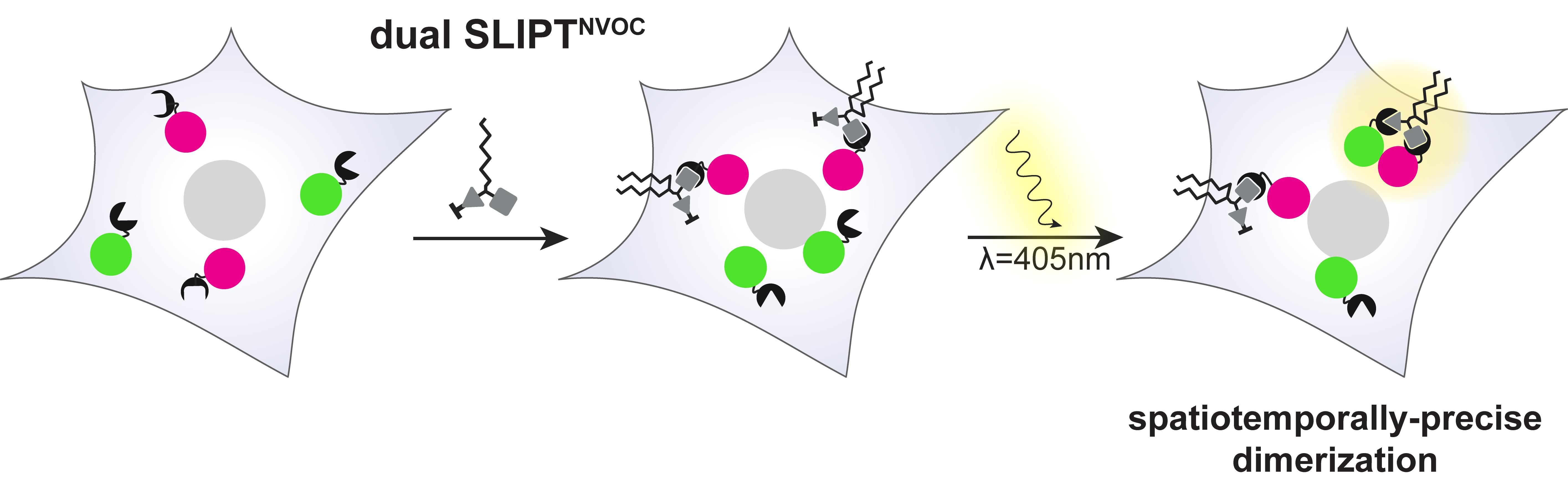
Figure 2. Mode of action of dual SLIPTNVOC. Cells expressing iK6eDHFR-POI A and HOB-POI B are treated with dual SLIPTNVOC. This causes PM recruitment of HOB-POI B inside the entire cell before irradiation of a subcellular region causes availability of the previously photocaged TMP arm and subsequent localized recruitment of iK6eDHFR-POI A. POI A and B are thereupon dimerized with high spatial and temporal precision.
Conventional CID (for reviews, see [5,6]) and optogenetic [7,8] strategies have been used to induce PM recruitment of POIs by prelocalizing one protein at the membrane (localizer) and translocating a second (actuator) upon stimulation (addition of the CID or irradiation with light). While suitable for constitutively membrane-associated POIs, these methods are less effective when both partners are cytosolic until signaling triggers their PM recruitment—a hallmark of many PM-associated signaling pathways, including many members of various actin signaling cascades (e.g., GEFs [9], GAPs [10], and nucleation-promoting factors [11]).
Chemically inducible trimerization (CIT) [12] addresses this limitation by enabling the recruitment of two cytosolic proteins to a membrane-localized third partner. However, similar to other CID and optogenetic systems, CIT requires more protein tags than actuators, increasing steric bulk and diverging from endogenous lipidation-mediated PM recruitment events.
Photocaged lipids (reviewed in [13]), on the contrary, offer the most native approach. They accumulate at the PM, being inactive until light-mediated uncaging, which reveals the active lipid headgroup and causes recruitment of proteins that can bind that specific lipid. While the most physiological approach mentioned here, manipulations with photocaged lipids are less orthogonal due to a lack of exact control over the identity of the recruited proteins. As a result, causality between PM recruitment of a specific POI and observed cellular effect is difficult to establish.
Dual SLIPT and dual SLIPTNVOC offer a compelling combination of the CID approach and the controlled release of endogenous lipids. The self-localizing nature of the lipid-like anchoring motif dispenses with the sterically demanding localizer, while maintaining the precision over POI identity and “plug-and-play” flexibility of traditional CID tools. The additional control over recruitment sequence, combined with the high spatiotemporal control of dual SLIPTNVOC, enables various experimental designs, such as engineering synthetic signaling pathways or the creation of logic circuits at the membrane.
This protocol describes the detailed synthesis, application, and validation of the two final probes, dual SLIPT and dual SLIPTNVOC. We describe how Trimethoprim and HaloTag ligand, both commercially available, are linker-functionalized to append them to a lipid-like anchoring motif. The said anchoring motif can be assembled by facile solid phase peptide synthesis (SPPS). This protocol further describes the use and rationale of various protecting groups that enable synthetic access to the final probes. To complete this protocol, minimal chemical training, as well as standard chemical equipment, is required. Possession of a peptide synthesizer is optional, but advisable.
Materials and reagents
All catalog numbers provided below shall serve as guide; alternative sources may be used as well.
Biological materials
1. HeLa (RRID:CVCL_0030) (DSMZ, catalog number: ACC 57) (see General note 1)
2. Halo-based oligonucleotide binder (Addgene, catalog number: 167268)
3. iK6eDHFR (Addgene, catalog numbers: 172100 and 172101)
Reagents
1. Boc-HaloTag ligand (ABCR, CAS number: 920264-35-3, catalog number: AB632176)
2. Trimethoprim (TCI, CAS number: 738-70-5, catalog number: T2286)
3. Myristic acid (TCI, CAS number: 544-63-8, catalog number: M0476)
4. Fmoc-D-Cysteine (Mmt) (AmBeed, CAS number: 1198791-73-9, catalog number: A311982)
5. Fmoc-8-amino-3,6-dioxaoctanoic (Roth, CAS number: 166108-71-0, catalog number: 7400.1)
6. Fmoc-Lys(ivDde) (Novabiochem, CAS number: 204777-78-6, catalog number: 8520820001)
7. Fmoc-Lys(Dde) (TCI, CAS number: 150629-67-7, catalog number: F1270)
8. Fmoc-4-aminobutyric acid (TCI, CAS number: 116821-47-7, catalog number: F0911)
9. Sieber amide resin (Novabiochem, CAS number: 915706-90-0, catalog number: 8550080001)
10. HBraq (TCI, CAS number: 10035-10-6, catalog number: H1220)
11. Ethyl-bromovalerate (TCI, CAS number: 14660-52-7, catalog number: B1557)
12. ((Nitroveratryl)oxy)chlorocarbamate (Aldrich, CAS number: 42855-00-5, catalog number: 420069-1G)
13. Glutaric anhydride (Aldrich, CAS number: 108-55-4, catalog number: G3806-5G)
14. 60% hydrazine monohydrate solution (TCI, CAS number: 7803-57-8, catalog number: H0172)
15. Diisopropylcarbodiimide (DIC) (TCI, CAS number: 693-13-0, catalog number: D0254)
16. Oxyma Pure (Aldrich, catalog number: 8510860100)
17. Piperidine (Roth, catalog number: A122.2)
18. Hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) (TCI, catalog number: A1797-5G)
19. Benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) (TCI, catalog number: 11450788)
20. Triisopropylsilane (TIS) (Fisher Scientific, catalog number: 11322157)
21. Dimethylformamide (DMF) (Roth, catalog number: A529.1)
22. DMF (peptide synthesis-grade) (Fisher Scientific, catalog number: 10387221)
23. DMF, anhydrous (Fisher Scientific, catalog number: 16398227)
24. 1-methyl-2-pyrrolidinone (Fisher Scientific, catalog number: 11360358)
25. Imidazole (Fisher Scientific, catalog number: 11369959)
26. Hydroxylamine hydrochloride (TCI, catalog number: H1581-25G)
27. Cesium carbonate (Cs2CO3) (Roth, catalog number: 6873.4)
28. Methanol (MeOH) (Fisher Scientific, catalog number: 30280932)
29. Dichloromethane (DCM) (Fisher Scientific, catalog number: 15538744)
30. DCM, anhydrous (TCI, catalog number: D3478-500ML)
31. N’, N’-Diisopropylethylamine (DIPEA) (Roth, catalog number: 4105.1)
32. Tetrahydrofuran (THF) (Thermo Scientific Alfa Aesar, catalog number: 11378016)
33. Lithium hydroxide (LiOH) (TCI, catalog number: L0225-25G)
34. Sodium hydroxide (NaOH), 2 M solution (Aldrich, catalog number: 1091361003)
35. Hydrochloric acid (HCl), 1 M solution (Aldrich, catalog number: 1603271003)
36. Trifluoracetic acid (TFA) (Fisher Chemical, catalog number: 10155347)
37. Formic acid (FA) (Fisher Scientific, catalog number: 30280925)
38. Acetonitrile (ACN) (Aldrich, catalog number: 60004-2.5L)
39. Triethylamine (Thermo Scientific Alfa Aesar, catalog number: 11489046)
40. 4-(Dimethylamino)pyridine (DMAP) (Aldrich, catalog number: 8510550100)
41. Deuterated methanol (CD3OD) (Aldrich, catalog number: 444758-10X1ML)
42. Deuterated dimethyl sulfoxide (DMSO-d6) (Aldrich, catalog number: 151874-10X0.75ML)
43. Deuterated chloroform (CDCl3) (Aldrich, catalog number: 151858-10X1ML)
44. Dimethyl sulfoxide (DMSO) (Fischer Scientific, catalog number: 11401611)
45. Lipofectamine 3000 (Thermo Fisher Scientific, catalog number: L3000008)
46. Phenol red-free DMEM (Gibco, catalog number: 31053-028)
47. High glucose DMEM + GlutaMAXTM (Gibco, catalog number: 31966-021)
48. OptiMEM (Thermo Fisher Scientific, catalog number: A4124802)
49. L-glutamine (Thermo Fisher Scientific, catalog number: 25030024)
50. Sodium pyruvate (Thermo Fisher Scientific, catalog number: 11360070)
51. Fetal bovine serum (FBS) (Fisher Scientific, catalog number: 15553681)
Solutions
1. Final peptide cleavage solution (see Recipes)
2. Fmoc-deprotection solution (see Recipes)
3. ivDde-deprotection solution (see Recipes)
4. Dde-deprotection solution (see Recipes)
5. Full DMEM (-) (see Recipes)
6. Full DMEM (-), phenol red-free (see Recipes)
7. Full DMEM (+) (see Recipes)
Recipes
1. Final peptide cleavage solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DCM | 93% v/v | 930 μL |
| TFA | 5% v/v | 50 μL |
| TIS | 2% v/v | 20 μL |
| Total | n/a | 1,000 μL |
Prepare freshly every time. The preparation must be conducted inside a ventilated fume hood.
2. Fmoc-deprotection solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMF | 80% v/v | 800 μL |
| Piperidine | 20% v/v | 200 μL |
| Total | n/a | 1,000 μL |
Piperidine in solution is stable for up to one month at 4 °C.
3. ivDde-deprotection solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMF | 95% v/v | 950 μL (12.3 mmol) |
| 60% Hydrazine monohydrate solution | 5% v/v | 50 μL |
| Total | n/a | 1,000 μL |
Prepare freshly every time. The preparation must be conducted inside a ventilated fume hood.
Caution: 60% Hydrazine monohydrate solution is flammable, toxic if swallowed, damages skin and eyes, potentially cancerous, and fatal if inhaled. The reaction must be conducted inside a ventilated fume hood.
4. Dde-deprotection solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1-methyl-2-pyrrolidinone | 8.7 M | 1 mL (10.4 mmol) |
| Imidazole | 0.104 M | 8.51 mg (125 μmol) |
| Hydroxylamine hydrochloride | 0.125 M | 10.4 mg (150 μmol) |
| DCM | 2.61 M | 200 μL (3.13 mmol) |
| Total | n/a | 1,200 μL/0.1 mmol resin |
Can be prepared for multiple reactions and stored for 1 month at 4 °C (without DCM).
Immediately prior to the deprotection, dilute the cocktail 5/1 (v/v) with DCM (1 mL of Dde-deprotection cocktail, 0.2 mL of DCM) for better efficiency of polystyrene-type resin deprotection.
The preparation must be conducted inside a ventilated fume hood.
Caution: 1-methyl-2-pyrrolidinone is a known irritant of skin and eyes, has reproductive toxicity, and damages the lungs upon single exposure. The reaction must be conducted inside a ventilated fume hood.
5. Full DMEM (-)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| High glucose DMEM + GlutaMAXTM | n/a | 495 mL |
| Sodium pyruvate | 1 mM | 5 mL |
| Total | n/a | 500 mL |
Store at 4 °C. The preparation must be conducted under sterile conditions.
6. Full DMEM (-), phenol red-free
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Phenol red-free DMEM | n/a | 490 mL |
| L-glutamine | 2 mM | 5 mL |
| Sodium pyruvate | 1 mM | 5 mL |
| Total | n/a | 500 mL |
Store at 4 °C. The preparation must be conducted under sterile conditions.
7. Full DMEM (+)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Full DMEM (-) | n/a | 450 mL |
| Heat-inactivated sterile-filtered FBS | 10% v/v | 50 mL |
| Total | n/a | 500 mL |
Store at 4 °C. The preparation must be conducted under sterile conditions.
Laboratory supplies
1. Personal protective equipment (the person performing the experiment must wear personal protective equipment, including goggles and a lab coat)
2. Magnetic stirrer (Heidolph, catalog number: 505-30000-00-4) and magnetic stir bars, various sizes (Fisher Scientific, catalog numbers: 16390656, 16380656, 16320666)
3. Oil BM2 viscosity bath media (Fisher Scientific, catalog number: 16020172)
4. pH-indicator strips MColorpHastTM (Merck, catalog number: 1.09535.0001)
5. Thin-layer chromatography POLYGRAM® SIL G/UV254 (Macherey-Nagel, catalog number: 805021)
6. UV lamp to visualize the TLC result (Herolab, catalog number: H468.1)
7. 0.3 mL low adsorption vial for HRMS (Supelco Analytical, catalog number: 29661-U)
8. 0.1 mL vial for LCMS (Carl Roth, catalog number: C518.1) with screwcaps (Carl Roth, catalog number: E161.1) and silicone/PTFE septum (Carl Roth, catalog number: E165.1)
9. NMR tubes with coded CLOSED caps (Bruker BioSpin, catalog number: Z172600)
10. Glassware and supplies: round-bottom flasks (VWR International GmbH, catalog numbers: SCOT241701307, SCOT241703702, SCOT2417011307; sizes: 25, 250, and 500 mL); pointed flasks (DWK Life Sciences, catalog numbers: 241950809, 241951402; sizes: 5 and 25 mL); Schlenk flask (Fisher Scientific, catalog number: 15884894); Erlenmeyer flask (Fisher Scientific, catalog number: 10840861)
11. Vials for storage 20 mL (Carl Roth, catalog number: LC88.1) and screwcaps (Carl Roth, catalog number: LC96.1)
12. Büchner funnel (Fisher Scientific, catalog number: 10771752)
13. Filter paper circles (Whatman, catalog number: 10311809)
14. Septum, mixed sizes (Aldrich, catalog number: Z124400)
15. PTFE filters (FisherbrandTM, catalog number: 11705285)
16. Disposables: plastic syringes (Henke Sass Wolf, catalog numbers: 4020-000V0, 4010-200V0), cannulae (Sterican®, catalog number: 4665643)
17. Microcentrifuge tubes (Eppendorf, catalog number: 15128344)
18. Amber-colored microcentrifuge tubes (Eppendorf, catalog number: 0030121155)
19. Fritted syringe: PP reactor 20 mL with PE frit, disposable (Multisyntech GmbH, catalog number: V200PE100)
20. Fritted syringe: PP reactor 5 mL with PE frit, disposable (Multisyntech GmbH, catalog number: V050PE063)
21. 50 mL Polypropylene centrifuge tubes (CEM Corp., catalog number: 330090)
22. Syringe pressure caps (Sigma-Aldrich, catalog number: Z120979-100EA)
23. µ-Slide 18-well glass bottom dishes (Ibidi, catalog number: 81817)
Equipment
1. Ventilated fume hood for chemistry (Waldner Laboreinrichtungen BmbH & Co.KG, model: SCALA)
2. Dual-manifold Schlenk line (Merck, catalog number: SYNM181004)
3. Cooling traps (Gaßner Glastechnik GmbH, catalog number: 5.3550.70)
4. Vacuum pump (Vacuubrand®, model: RC6)
5. Heat gun (Steinel Professional, model: 3515)
6. Dewar (KGW Isotherm, catalog number: 10558672)
7. Mass spectrometry for characterization of compounds (Bruker, model: maXis IITM ETD-HRMS)
8. 1H NMR for characterization of compounds (Bruker, model: Advance III HD 400 NMR), equipped with a CryoProbeTM
9. HPLC for the purification of compounds (Thermo Scientific, model: UltiMate300 UHPLC)
10. C18 column for small molecule purification [Merck, Ascentis® C18 (5 μm) HPLC Column (25 × 212 nm), catalog number: 5811347-U]
11. C4 column for lipo-peptide purification [HiChrom, Vydac 214TP 10 μm C4 column (22 × 250 mm), catalog number: HI-214TP1022]
12. Liquid chromatography mass spectrometry (LC-MS) (Shimadzu, model: MS20-20)
13. C18 column for LCMS (Merck, TitanTM C18 80 Å 1.9 μm, 2.1 × 50 mm, catalog number: 577122-U)
14. Flash chromatography (Biotage®, model IsoleraTM One with UV-Vis Detector, part number: ISO-1EW)
15. Flash chromatography columns (Supelco®, model: SupelTM Flash Cartridge, 40 g, 40–60 μm silica, catalog number: FSISI040)
16. Rotary evaporator (Heidolph, catalog number: 571-01300-00)
17. Lyophilizer (Christ, model: Alpha 2–4 LDplus)
18. Peptide synthesizer (CEM Corp., model: Liberty BlueTM)
19. H12 resin loader (CEM Corp., catalog number: 909912)
20. 20 L stainless steel bottle with sight tube (CEM Corp., catalog number: 551255)
21. 2 L stainless steel bottle with sight tube (CEM Corp., catalog number: 551240)
22. 1 L safety coated glass bottle (CEM Corp., catalog number: 551330)
23. 500 mL safety coated glass bottle (CEM Corp., catalog number: 551325)
24. 30 mL reaction vessel (CEM Corp., catalog number: 167260)
25. Microscope (Leica, model: SP8 LIGHTNING Super-resolution live cell imaging in multicolor) equipped with white-light laser and FRAP module
26. Power meter (Thorlabs, catalog number: PM400)
27. Microscope slide power meter sensor heads (Thorlabs, catalog number: S170C)
Software and datasets
1. ImageJ (Wayne Rasband and contributors NIH, USA, version: 1.54p), free to use
2. Leica Application Suite X (LAS X) Microscopy Software (Leica, version: 3.5.7.23225), requires a license
3. Excel (Microsoft® Office, version 2108), requires a license
4. GraphPad Prism (Software MacKievTM, version: 10.5.0 (774), May 27, 2025), requires a license
5. Liberty Blue Application Software (CEM Corp., version: 1.50.5913.17379, March 10, 2016)
Procedure
文章信息
稿件历史记录
提交日期: Jul 30, 2025
接收日期: Sep 16, 2025
在线发布日期: Oct 17, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bayer, K. V. and Wombacher, R. (2025). Lipid-Mediated Sequential Recruitment of Proteins Via Dual SLIPT and Dual SLIPTNVOC in Live Cells. Bio-protocol 15(21): e5489. DOI: 10.21769/BioProtoc.5489.
分类
生物化学 > 脂质 > 脂质-蛋白互作
生物化学 > 蛋白质 > 相互作用 > 蛋白质-脂质相互作用
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












