- EN - English
- CN - 中文
Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5487 浏览次数: 1448
评审: Joyce Chiu
Abstract
This protocol describes the isolation and flow cytometric analysis of extracellular vesicles (EVs) derived from red blood cells, endothelial cells, and platelets in human peripheral blood. The protocol includes steps for preparing platelet-free plasma, fluorescent antibody staining, gating strategies, and technical controls. This protocol was developed within a study on EV release in snakebite-associated thrombotic microangiopathy; the protocol addresses challenges such as variable autofluorescence and heterogeneity in EV origin. It is flexible and can be adapted for alternative antibody panels targeting different cell populations or EV subtypes, including leukocyte-derived EVs.
Key features
• Bead-free, two-step plasma preparation enhances extracellular vesicle yield, reduces platelet contamination, and improves purity compared with conventional isolation methods for small-volume clinical samples.
• Reduced autofluorescence by compensation strategy using flow cytometry.
• Gating strategies to detect distinct EV populations derived from red cells, endothelial cells, and platelets.
• Validated in healthy donors and patients, enabling reproducible detection of EVs with broad downstream compatibility for flow cytometric applications.
Keywords: Bead-Free (无磁珠法)Graphical overview
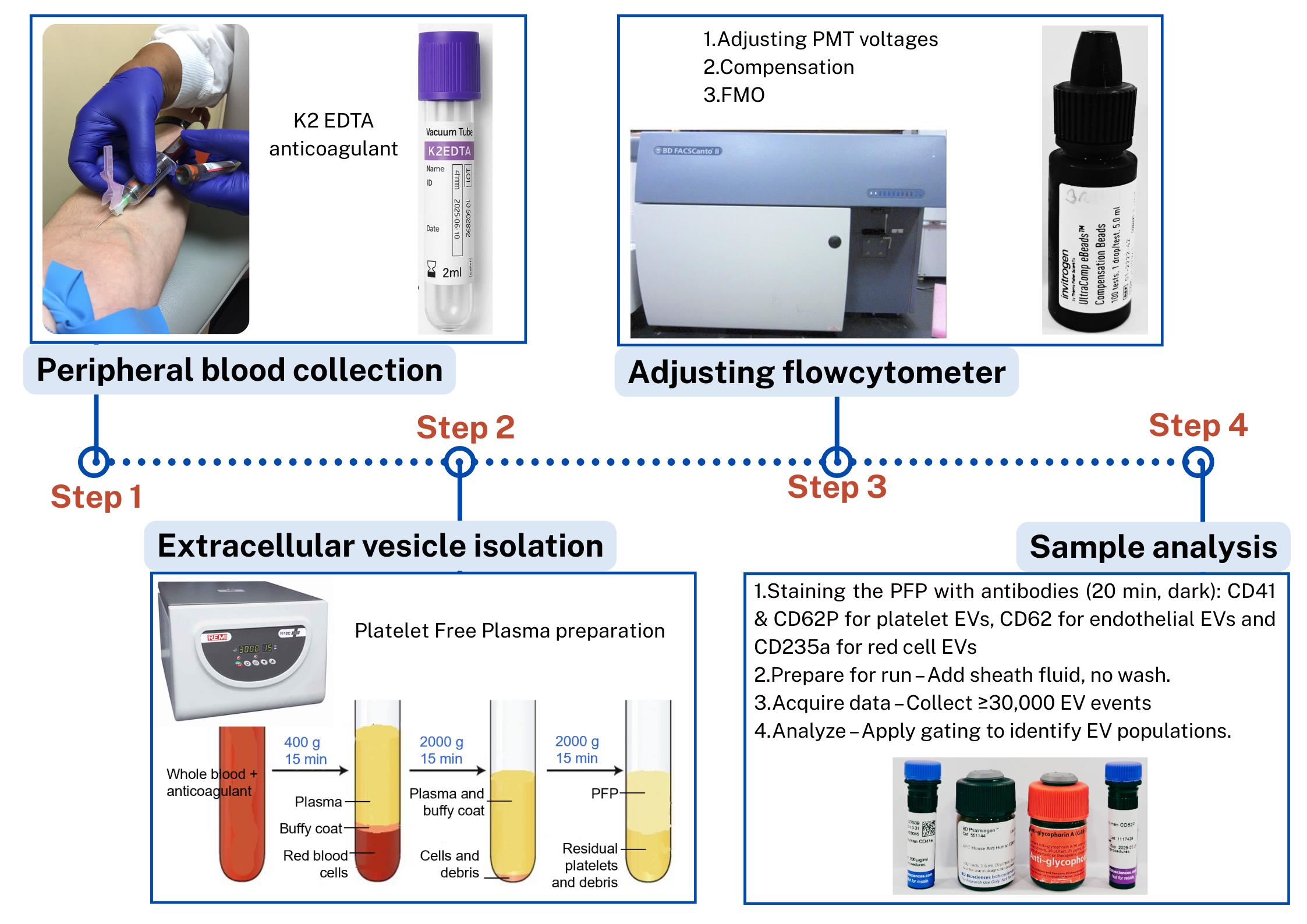
Steps in the isolation and flow cytometric analysis of extracellular vesicles (EVs) derived from red blood cells, endothelial cells, and platelets in human peripheral blood
Background
This protocol outlines detailed steps for isolating extracellular vesicles (EVs) from human peripheral blood and characterizing them using flow cytometry [1]. It was developed as part of a study investigating EV release in patients with snakebite-associated thrombotic microangiopathy.
EVs play important roles in health and disease, but their small size, heterogeneity, and variable autofluorescence make analysis challenging [2]. Several techniques have been developed for EV analysis, including electron microscopy, nanoparticle tracking analysis, tunable resistive pulse sensing, western blotting, and ELISA-based assays, each with advantages and limitations [1]. Existing EV isolation protocols, including ultracentrifugation, precipitation, and size-exclusion chromatography, often face challenges such as incomplete removal of contaminating platelets, co-isolation of protein aggregates, variable EV recovery, and loss of vesicles during multiple washing steps [3]. Moreover, differences in centrifugation speeds, buffer preparation, and gating strategies limit reproducibility across laboratories [4]. Flow cytometry–based detection adds further complexity due to EV heterogeneity, variable autofluorescence, and the difficulty of distinguishing true EVs from debris [5].
Bead-based analysis of EVs is commonly used to overcome the challenge of their small size, which limits direct detection by conventional flow cytometry. In this approach, EVs are captured onto larger beads (typically 4–6 μm) coated with antibodies against tetraspanins such as CD63, CD81, or CD9, thereby increasing their effective size and enabling detection and characterization by flow cytometry. Once bound, the bead–EV complexes can be further probed with fluorescent antibodies to analyze additional surface markers and identify subpopulations. While this method is practical and widely accessible, it has important limitations: it selectively enriches only those EVs expressing the capture antigen (e.g., CD63+ vesicles if anti-CD63 beads are used), potentially overlooking other populations, and multiple EVs may attach to a single bead, preventing true single-vesicle resolution. Consequently, bead-based analysis provides useful phenotyping information but does not fully represent the heterogeneity of circulating EVs [6,8].
Our protocol addresses these issues by providing a standardized platelet-free plasma preparation method to minimize platelet contamination, with no unnecessary washing steps to reduce EV loss, use of filtered buffers to decrease background noise, and detailed stepwise explanations with troubleshooting guidance to improve reproducibility. Importantly, the protocol is a bead-free, flexible approach that can be adapted for different antibody panels and EV subtypes, enhancing its applicability compared with many existing protocols.
Materials and reagents
Biological materials
1. Human whole blood samples
Note: This study was approved by the Ethics Review Committee of the Faculty of Medicine, University of Colombo, Sri Lanka, EC-22-080).
Reagents
1. Mouse anti-human glycophorin A [GAR-2(HIR-2)] or CD235a, PE (25 μg/mL) (BD Biosciences, catalog number: 340947)
2. Mouse anti-human CD62E (68-5H11), APC (25 μg/mL) (BD Biosciences, catalog number: 551144)
3. Mouse anti-human CD62P (AK-4), BV421 (25 μg/mL) (BD Biosciences, catalog number: 564038)
4. Mouse anti-human CD41a (HIP8), PE-CyTM7 (200 μg/mL) (BD Biosciences, catalog number: 5641424)
5. UltraComp eBeadsTM compensation beads (ThermoFisher Scientific, catalog number: 01-2222-41)
6. BD FACSDiva CS&T research beads (BD Biosciences, catalog number: 655050)
7. BD OneFlow setup beads (BD Biosciences, catalog number: 658620)
8. FACS clean solution, 5 L (BD Biosciences, catalog number: 340345)
9. FACS shutdown solution, 5 L (BD Biosciences, catalog number: 334224)
10. FACS flow sheath, 20 L (BD Biosciences, catalog number: 342003)
11. Sodium chloride (NaCl)
12. Potassium chloride (KCl)
13. Disodium hydrogen phosphate, anhydrous (Na2HPO4)
14. Potassium dihydrogen phosphate (KH2PO4)
15. Phosphate buffer saline (1×), pH 7.4 (Thermo Fisher Scientific, catalog number: 10010023)
Laboratory supplies
1. Plastic round bottom tubes 3 mL (Plastica International Pvt. Ltd., Sri Lanka)
2. Falcon® round bottom polystyrene tubes (FACS tubes) (Corning Life Sciences, catalog number: 352054)
3. Micropipette tips: 5 μL, 200 μL, 1,000 μL (BIOLOGIX, China)
4. Microcentrifuge tube (1.5 mL) (BIOLOGIX, China)
5. 3 mL disposable syringe with 23-gauge needle (Changzhou Medical appliances, China)
6. K2-EDTA vacutainer tubes [CML biotech (Pvt) Ltd., Sri Lanka]
7. Filters with pore size 0.2 μm
Equipment
1. BD FACSCantoTM II Flow Cytometer (BD Biosciences, USA, catalog number: R33896203257)
2. REMI R-8C PLUS centrifuge (Remi Elektrotechnik Ltd., India, catalog number: ZGEN-10144)
3. Vortex mixer (Remi Elektrotechnik Ltd., India)
4. Refrigerator (2–8 °C)
Software and datasets
1. BD FACSDiva Software (BD Biosciences, USA, version 8.0.3)
2. FlowJoTM (BD Biosciences, USA, version 10.0)
Procedure
文章信息
稿件历史记录
提交日期: Aug 5, 2025
接收日期: Sep 23, 2025
在线发布日期: Oct 11, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Alvitigala, B. Y., Wijewickrama, E. S., Denney, L., Weeratunga, P., Kaluarachchi, P., Gnanathasan, A. and Gooneratne, L. V. (2025). Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles. Bio-protocol 15(21): e5487. DOI: 10.21769/BioProtoc.5487.
分类
细胞生物学 > 细胞器分离 > 胞外囊泡
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











