- EN - English
- CN - 中文
Colocalizing Telomeres With PML or γH2AX Foci by IF-FISH in Mouse Brain Neurons
利用 IF-FISH 技术在小鼠脑神经元中检测端粒与 PML 或 γH2AX 焦点的共定位
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5485 浏览次数: 1515
评审: Elena A. OstrakhovitchAnonymous reviewer(s)
Abstract
Telomere length maintenance is strongly linked to cellular aging, as telomeres progressively shorten with each cell division. This phenomenon is well-documented in mitotic, or dividing, cells. However, neurons are post-mitotic and do not undergo mitosis, meaning they lack the classical mechanisms through which telomere shortening occurs. Despite this, neurons retain telomeres that protect chromosomal ends. The role of telomeres in neurons has gained interest, particularly in the context of neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), where aging is a major risk factor. This has sparked interest in investigating telomere maintenance mechanisms in post-mitotic neurons. Nevertheless, most existing telomere analysis techniques were developed for and optimized using mitotic cells, posing challenges for studying telomeres in non-dividing neuronal cells. Thus, this protocol adapts an already established technique, the combined immunofluorescence and telomere fluorescent in situ hybridization (IF-FISH) on mitotic cells to study the processes occurring at telomeres in cortical neurons of the mouse ALS transgenic model, TDP-43 rNLS. Specifically, it determines the occurrence of DNA damage and the alternative lengthening of telomeres (ALT) mechanism through simultaneous labeling of the DNA damage marker, γH2AX, or the ALT marker, promyelocytic leukemia (PML) protein, together with telomeres. Therefore, the protocol enables the visualization of DNA damage (γH2AX) or the ALT marker (PML) concurrently with telomeres. This technique can be successfully applied to brain tissue and enables the investigation of telomeres specifically in cortical neurons, rather than in bulk tissue, offering a significant advantage over Southern blot or qPCR-based techniques.
Key features
• This protocol enables the labeling of telomeres in mouse brain tissue prepared from paraffin-embedded brain sections.
• This method facilitates concurrent labeling of proteins that are colocalized at telomere sites.
Keywords: IF-FISH (IF-FISH)Graphical overview
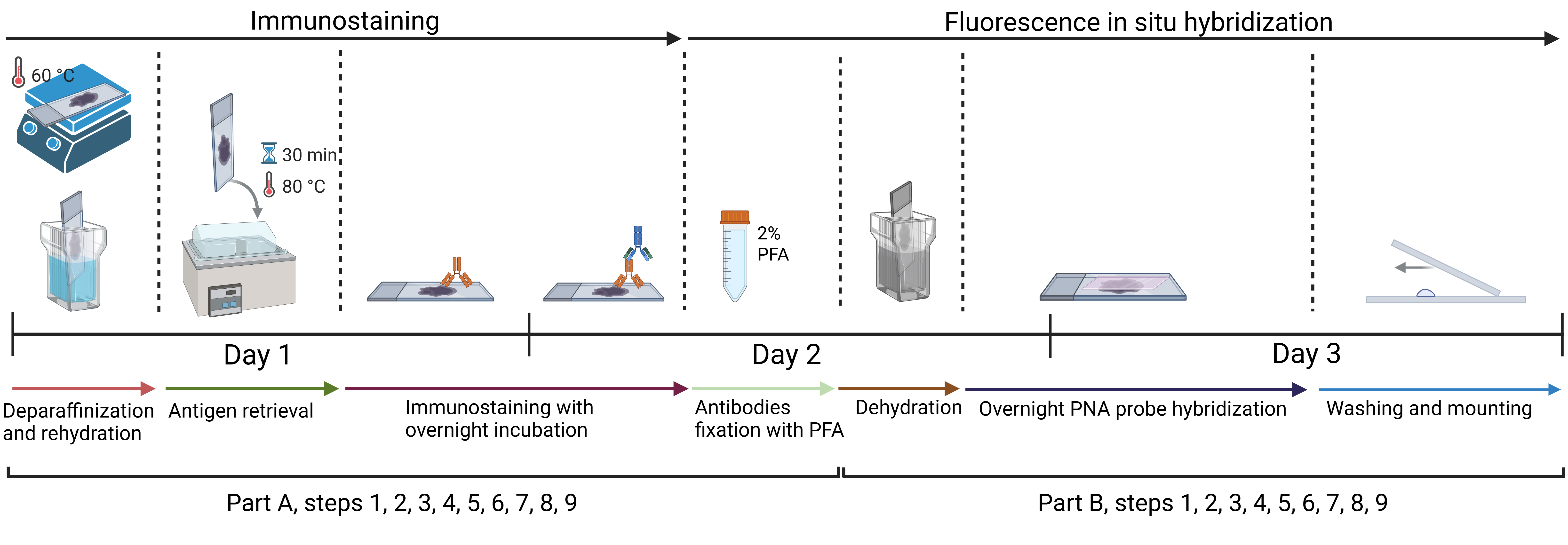
Approximate timeline for conducting immunofluorescence and telomere fluorescent in situ hybridization (IF-FISH)
Background
This protocol has the potential to advance the study of telomere length maintenance mechanisms, particularly in neurons of transgenic mouse models of neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS). As post-mitotic cells, neurons do not undergo cell division [1]; therefore, telomere homeostasis is likely regulated differently in these cells compared to mitotic cells, in which telomeres progressively shorten with each round of replication [2]. Telomere length maintenance represents an emerging and compelling area of research, especially in the context of pathological conditions such as ALS that affect the nervous system [3,4].
Established methods for studying telomeres include Southern blot analysis, which enables measurement of terminal restriction fragment (TRF) length [5], and quantitative PCR (qPCR)-based techniques [6], which estimate relative telomere length by comparing telomeric repeat copy number to that of a single-copy gene. While these methods are useful, they often lack cell-type specificity and are not well-suited for analyzing telomere dynamics in heterogeneous tissues such as the brain. ALS affects specific neuronal subtypes in the brain and spinal cord [7]; therefore, a method that enables telomere analysis within the desired cell type is particularly valuable for improving the understanding of the disease mechanism. Moreover, these techniques do not allow for the investigation of molecular events at telomeres, e.g., telomere dysfunction-induced foci (TIFs), and the occurrence of DNA damage or the recruitment of telomere-associated proteins.
In contrast, the described technique for immunofluorescence combined with fluorescence in situ hybridization (IF-FISH) enables the visualization and analysis of telomeric or telomere-associated proteins at telomeric sites, such as TRF2 or Rif1, thereby providing deeper insights into telomere-related molecular processes. This method is particularly valuable in studies requiring cell-type specificity and spatial resolution, making it especially suitable for complex tissues like the brain. While IF-FISH is less suitable for precise telomere length quantification compared to methods such as Southern blot or qPCR, it nonetheless allows for the evaluation of relative telomere length based on fluorescence intensity. Thus, although this approach does not yield exact telomere length measurements, it is still valuable for detecting and comparing telomere length differences between experimental groups. Furthermore, IF-FISH provides spatial information at the single-cell level. When combined with quantitative data from methods such as qPCR or Southern blotting, it enhances the overall interpretation by offering critical context regarding cell-to-cell heterogeneity and the spatial organization of telomeres. This integrative approach enables a more comprehensive and multidimensional analysis of telomere dynamics under various experimental conditions. However, the limitation of the IF-FISH method is its relatively time-consuming protocol, particularly when compared to qPCR-based approaches.
Materials and reagents
Biological materials
1. Mouse brain paraffin sections, 10 µm thick (derived from transgenic bigenic rNLS mouse or monogenic control mouse [8])
Reagents
1. Trisodium citrate·2H2O (Merck, catalog number: 1064480500)
2. Tween-20 (Merck, catalog number: 11332465001)
3. Normal donkey serum (NDS) (Jackson ImmunoResearch, catalog number: 017-000-121)
4. Histone H2AX [p Ser139] antibody (Novus Biologicals, catalog number: NB100-384)
5. Anti-PML antibody, mouse monoclonal (Merck-Millipore, catalog number: P6746-200UL)
6. Tris base (Merck, catalog number: 252859-500G)
7. Sodium chloride (Merck, catalog number: S9625-1KG)
8. Potassium chloride (Merck, catalog number: P3911-500G)
9. Sodium phosphate dibasic (Merck, catalog number: S9763-500G)
10. Potassium phosphate monobasic (Merck, catalog number: P0662-500G)
11. Xylene (histological grade), (Merck, catalog number: 534056-500ML)
12. Alexa FluorTM 488, anti-mouse (Invitrogen, catalog number: A-21202)
13. Alexa FluorTM 488, anti-rabbit (Invitrogen, catalog number: A-21206)
14. Paraformaldehyde, 16% (Thermo Fisher Scientific, catalog number: 043368.9M)
15. Texas Red-conjugated C-strand telomere PNA probe (stock solution of 0.3 μL/mL in Mili-Q water) (Panagene, custom fluorescent dye)
16. Blocking reagent (Merck, catalog number: 11096176001)
17. Formamide (Merck, catalog number: F7503-100ML)
18. Hydrochloric acid (Merck, catalog number: 258148-25ML)
19. DAPI (Merck, catalogue number: D9542-1MG)
Solutions
1. 10 mM citrate buffer containing 0.1% Tween-2, pH 6.0 (see Recipes)
2. 1× PBS, pH 7.4 (see Recipes)
3. 1× PBS, pH 7.4 with 0.1% Triton X-100 (see Recipes)
4. 5% NDS (see Recipes)
5. Histone H2AX [p Ser139] antibody 1:200 dilution (see Recipes)
6. Anti-PML antibody 1:200 dilution (see Recipes)
7. 90% ethanol (see Recipes)
8. 70% ethanol (see Recipes)
9. 10 mM Tris Cl, pH 7.5 (see Recipes)
10. 50 mM Tris Cl, pH 7.5 (see Recipes)
11. PNA wash buffer A (70% v/v formamide, 10 mM Tris Cl, pH 7.5) (see Recipes)
12. PNA wash buffer B (50 mM Tris Cl, pH 7.5, 150 mM NaCl, 0.8% v/v Tween 20) (see Recipes)
13. 2% paraformaldehyde in 1× PBS (see Recipes)
14. PNA hybridization solution (see Recipes)
15. PNA probe hybridization working solution (see Recipes)
Recipes
1. 10 mM citrate buffer containing 0.1% Tween-20, pH 6.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Trisodium citrate·2H2O (MW = 294.10 g/mol) | 10 mM | 2.94 g |
| Milli-Q water | - | To 1,000 mL total volume |
| Tween-20 | 0.1% v/v | 1.0 mL |
| Total | 1,000 mL |
If necessary, adjust to pH 6.0 with HCl.
2. 1× PBS, pH 7.4
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride (MW = 58.44 g/mol) | 137 mM | 8 g |
| Potassium chloride (MW = 74.55 g/mol) | 2.7 mM | 0.2 g |
| Sodium phosphate dibasic (MW = 141.96 g/mol) | 10 mM | 1.44 g |
| Potassium phosphate monobasic (MW = 136.09 g/mol) | 1.8 mM | 0.245 g |
| Milli-Q water | - | To 1,000 mL total volume |
| Total volume | 1,000 mL |
Adjust to pH 7.4 with HCl.
3. 1× PBS, pH 7.4 with 0.1% Triton X-100
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride (MW = 58.44 g/mol) | 137 mM | 8 g |
| Potassium chloride (MW = 74.55 g/mol) | 2.7 mM | 0.2 g |
| Sodium phosphate dibasic (MW = 141.96 g/mol) | 10 mM | 1.44 g |
| Potassium phosphate monobasic (MW = 136.09 g/mol) | 1.8 mM | 0.245 g |
| Milli-Q water | - | To 1,000 mL total volume |
| Triton X-100 | 0.1% v/v | 1.0 mL |
| Total | 1,000 mL |
Adjust to pH = 7.4 with HCl.
4. 5% NDS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Normal donkey serum | 5% | 0.5 mL |
| 1× PBS with 0.1% Triton X-100 | - | 9.5 mL |
| Total | 10 mL |
5. Histone H2AX [p Ser139] antibody 1:200 dilution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 5% NDS | 5% | 199 μL |
| Histone H2AX [p Ser139] antibody | 5 μg/mL | 1 μL |
| Total volume per one slide | 200 μL |
6. Anti-PML antibody 1:200 dilution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 5% NDS | 5% | 199 μL |
| Anti-PML antibody | 10–12.5 μg/mL | 1 μL |
| Total volume per one slide | 200 μL |
7. 90% ethanol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Absolute ethanol (100%) | 90% | 225 mL |
| Milli-Q water | - | 25 mL |
| Total | 250 mL |
8. 70% ethanol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Absolute ethanol (100%) | 70% | 175 mL |
| Milli-Q water | - | 75 mL |
| Total | 250 mL |
9. 10 mM Tris Cl, pH 7.5
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris base (MW = 121.14 g/mol) | 10 mM | 1.21 g |
| Milli-Q water | - | up to 1,000 mL |
| Total | 1,000 mL |
Adjust to pH 7.5 with HCl.
10. 50 mM Tris Cl, pH 7.5
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris base (MW = 121.14 g/mol) | 10 mM | 6.06 g |
| Milli-Q water | - | up to 1,000 ml |
| Total | 1,000 mL |
Adjust to pH 7.5 with HCl.
11. PNA wash buffer A (70% v/v formamide, 10 mM Tris Cl, pH 7.5)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 10 mM Tris Cl, pH 7.5 | - | 150 mL |
| Formamide | 70% | 350 mL |
| Total | 500 mL |
12. PNA wash buffer B (50 mM Tris Cl, pH 7.5, 150 mM NaCl, 0.8% v/v Tween 20)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 50 mM Tris Cl, pH 7.5 | 50 mM | 490 mL |
| Sodium chloride (58.44 g/mol) | 150 mM | 4.39 g |
| Tween-20 | 0.8% v/v | 4 mL |
| 50 mM Tris Cl, pH 7.5 | 50 mM | To 500 mL total volume |
| Total | 500 mL |
13. 2% paraformaldehyde in 1× PBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 16% paraformaldehyde | 2% | 1.25 mL |
| 1× PBS, pH 7.4 | 8.75 mL | |
| Total | 10 mL |
14. PNA hybridization solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 10 mM Tris Cl, pH 7.5 | - | 2.975 mL |
| Formamide | 70% | 7 mL |
| Blocking reagent | 0.25% | 25 μL |
| Total | 10 mL |
15. PNA probe hybridization working solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PNA hybridization solution | - | 999 μL |
| 0.3 μg/mL C-strand telomere PNA probe | 0.3 ng/mL | 1 μL |
| Total | 1,000 μL |
Laboratory supplies
1. Parafilm® M sealing film (Merck, catalog number: HS234526B-1EA)
2. Cover glasses (Merck, catalog number: C9802)
3. Coplin jars, e.g., BRAND® plastic staining trough, Hellendahl pattern (Merck, catalog number: BR474400)
4. Dark humidity chamber for slides, e.g., StainTray slide staining system (Merck, catalog number: Z670146-1EA)
5. ProLongTM Gold Antifade Mountant (ThermoFisher scientific, catalog number: P36930)
6. Deionized water
Equipment
1. Hot plate, e.g., benchmark hotplate (Merck, catalog number: Z742547)
2. Drying oven, e.g., binder multifunction drying oven FED series (Merck, catalog number: Z604011)
3. Water bath with heating, e.g., MyBathTM mini water bath (Merck, catalog number: Z741427)
Procedure
文章信息
稿件历史记录
提交日期: Aug 9, 2025
接收日期: Sep 25, 2025
在线发布日期: Oct 9, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Konopka, A. (2025). Colocalizing Telomeres With PML or γH2AX Foci by IF-FISH in Mouse Brain Neurons. Bio-protocol 15(21): e5485. DOI: 10.21769/BioProtoc.5485.
分类
神经科学 > 神经系统疾病 > 神经退行性病变
分子生物学 > DNA > DNA 损伤和修复
神经科学 > 基础技术 > 染色质生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












