- EN - English
- CN - 中文
Optogenetic Approach for Investigating Descending Control of Nociception in Ex Vivo Spinal Cord Preparation
利用光遗传学研究离体脊髓中疼痛下行调控机制
发布: 2025年11月05日第15卷第21期 DOI: 10.21769/BioProtoc.5483 浏览次数: 1920
评审: Anonymous reviewer(s)
Abstract
Nociception is critically shaped by descending modulation of spinal circuits, yet its cellular and synaptic mechanisms remain poorly defined. Elucidating these mechanisms is technically challenging, as it requires simultaneous activation of primary afferents and descending fibers while monitoring the functioning of individual spinal neurons. Here, we present a method to investigate the influence of the rostral ventromedial medulla (RVM), a principal supraspinal structure mediating descending modulation, on the activity of spinal lamina I neurons. Our approach combines electrophysiological recordings in ex vivo intact spinal cord preparation with optogenetics, granting several advantages. First, ex vivo preparation spares rostrocaudal and mediolateral spinal architecture, preserving lamina I as well as primary afferent and descending inputs. Second, virally mediated channelrhodopsin-2 (ChR2) expression enables selective photostimulation of RVM-originating fibers. When coupled with patch-clamp recordings, this photostimulation allows identifying postsynaptic inputs from RVM to spinal neurons and revealing RVM-dependent presynaptic inhibition of primary afferent inputs. Overall, our approach is well-suited for investigating both pre- and postsynaptic mechanisms of descending modulation in physiological and pathological pain conditions.
Key features
• Rapid preparation procedure that grants access to lamina I neurons while preserving spinal cord architecture, including primary afferents and descending inputs.
• Optogenetic approach allowing functional studies of RVM-dependent descending modulation.
• Ability to assess RVM fiber–dependent presynaptic inhibition of synaptic transmission between primary afferents and lamina I neurons.
Keywords: Optogenetics (光遗传学)Graphical overview
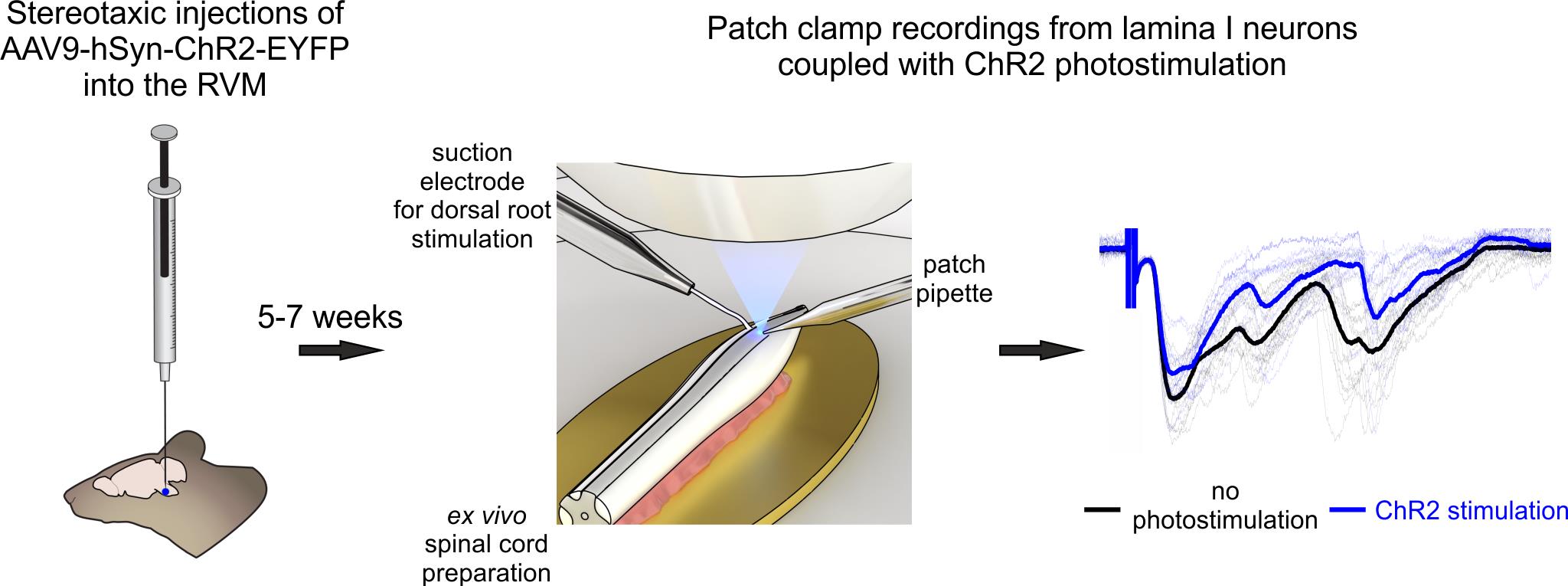
Optogenetic approach for functional studies of rostral ventromedial medulla (RVM)-dependent descending control of spinal lamina I neurons
Background
Neurons of the superficial dorsal horn (spinal laminae I–II) receive direct input from high-threshold primary afferents, perform primary processing of nociceptive signals, and relay pain-related information to the brain [1,2]. At the same time, this important ascending pathway is subject to descending feedback control. Several supraspinal structures located mostly in the hindbrain modulate the functioning of nociceptive-processing spinal neurons, thereby facilitating or inhibiting pain sensations [3–5]. Therefore, understanding the mechanisms of descending modulation is essential for advancing pain research.
Unfortunately, descending modulation remains poorly studied due to technical challenges that arise from the intrinsic organization of the central nervous system. Spinal and supraspinal structures are spatially separated (even in mice, they are a few centimeters apart), making it difficult to preserve both for functional studies. To address this challenge, several different approaches have been developed. The first one involves single-unit recordings from spinal neurons coupled with electrical stimulations of brainstem regions [6–8]. Although this method provides valuable information on how descending regulation affects action potential generation in spinal neurons, it offers no insight into its cellular and synaptic mechanisms. Besides, this method is difficult to apply to lamina I, as it is an extremely thin layer extending only about 30 μm from the surface of the dorsal horn. The second approach involves patch-clamp recordings in ex vivo spinal cord preparation combined with electrical stimulations of various descending spinal cord tracts [9]. While this technique is more suited for mechanistic studies, it cannot distinguish the impact of individual supraspinal structures, as their fibers largely travel within the same dorsolateral funiculus. Lastly, the third approach relies on optogenetics, i.e., virally mediated expression of light-sensitive channelrhodopsin-2 protein in the axons and terminals of descending fibers [10]. Combining patch-clamp recordings from superficial dorsal horn neurons with selective, photostimulation-induced activation of descending fibers originating from a single supraspinal source offers a unique possibility to determine how specific brain structures exert descending control. However, this method requires slicing the spinal cord, which damages lamina I and compromises primary afferent input—an important limitation, as descending fibers are known to modulate synaptic transmission between primary nociceptors and spinal neurons through presynaptic mechanisms.
Here, we provide a detailed protocol integrating patch-clamp recordings from lamina I neurons of intact ex vivo spinal cord preparation with optogenetic stimulation of descending fibers originating from rostral ventromedial medulla (RVM), a key hindbrain region mediating descending control. The main advantages of the proposed procedure are a) selective activation of RVM fibers, b) sparing the dendritic and axonal arborization of lamina I neurons, and c) preservation of the entire rostrocaudal and mediolateral spinal cord architecture, including all primary afferent input and RVM connections, important for studying both pre- and postsynaptic mechanisms of descending regulation. Compared to other methods that rely on slicing, ex vivo spinal cord preparation is faster and more reliable; the preparation is ready within 30–40 min after decapitation, it does not require any additional incubation, and may be used for up to 6–8 hours. Furthermore, cell visualization is performed using infrared LED oblique illumination [11,12], which does not require any sophisticated equipment for differential interference contrast.
Our protocol is well-suited for investigating RVM-dependent descending modulation under both physiological and pathological pain conditions. It can also be readily adapted to study descending control mediated by other supraspinal structures, such as the dorsal reticular nucleus and parabrachial nucleus. The electrophysiological recordings described here are compatible with pharmacological investigations and can be combined with Ca2+ transient measurements using either membrane-impermeable or genetically encoded Ca2+ indicators.
Since the protocol uses mice as the model organism, it is compatible with a wide range of transgenic lines, enabling flexible experimental design. In particular, animals expressing GFP in glutamatergic, GABAergic, or glycinergic neurons can help elucidate the mechanisms of descending modulation in distinct populations of spinal neurons. Lastly, retrograde labeling of lamina I projection neurons may offer valuable insights into how supraspinal structures influence the transmission of nociceptive signals to higher brain centers.
Materials and reagents
Biological materials
1. 12–15-week-old mice (any strain)
2. AAV9-hSyn-hChR2(H134R)-EYFP (titer ≥ 1 × 1013 vg/mL) (AddGene, catalog number: 26973-AAV9)
Reagents
1. Sucrose (Merck, catalog number: S0389)
2. Glucose (Merck, catalog number: G8270)
3. Sodium chloride (NaCl) (Merck, catalog number: S9888)
4. Sodium bicarbonate (NaHCO3) (Merck, catalog number: S0751)
5. Sodium monophosphate (NaH2PO4) (Merck, catalog number: S6040)
6. Potassium chloride (KCl) (Merck, catalog number: P9333)
7. Magnesium chloride (MgCl2·6H2O) (Merck, catalog number: M2670)
8. Calcium chloride (CaCl2·2H2O) (Merck, catalog number: C7902)
9. Potassium gluconate (Merck, catalog number: G4500)
10. Sodium ATP (Na2ATP) (Merck, catalog number: A3377)
11. Sodium GTP (NaGTP) (Merck, catalog number: G8877)
12. HEPES (Merck, catalog number: H3375)
13. EGTA (Merck, catalog number: E0396)
14. Sodium ascorbate (Merck, catalog number: 11140)
15. Sodium pyruvate (Merck, catalog number: P2256)
16. Potassium hydroxide (KOH) (Honeywell-Fluka, product number: 15653560)
17. Anesthetic agents: ketamine hydrochloride (50 mg/mL, Farmak) and sedazin (20 mg/mL, xylazine, Biowet Pulawi) or isoflurane (Abbvie, catalog number: B506)
18. Mineral oil (Merck, catalog number: M5904)
19. 95% O2 and 5% CO2 gas mixture (locally sourced)
20. O2 gas (in case isoflurane anesthesia is used for stereotaxic injections)
Solutions
1. Sucrose dissection solution (see Recipes)
2. Krebs bicarbonate solution (see Recipes)
3. Potassium gluconate intracellular solution (see Recipes)
Recipes
1. Sucrose dissection solution
| Reagent | Final concentration | Quantity |
|---|---|---|
| Sucrose | 200 mM | 34.23 g |
| Glucose | 11 mM | 991 mg |
| NaHCO3 | 26 mM | 1.092 g |
| NaH2PO4 | 1.2 mM | 72 mg |
| KCl | 2 mM | 74.5 mg |
| MgCl2·6H2O | 7 mM | 712 mg |
| CaCl2·2H2O | 0.5 mM | 37 mg |
| Sodium ascorbate (optional) | 5 mM | 495 mg |
| Sodium pyruvate (optional) | 3 mM | 168 mg |
| ddH2O | to 0.5 L |
pH is 7.3–7.4 when bubbled with 95% O2 and 5% CO2. Osmolarity is 310–320 mOsm/kg.
This solution may be used for up to 10 days after preparation if kept at 4–8 °C.
2. Krebs bicarbonate solution
| Reagent | Final concentration | Quantity |
|---|---|---|
| NaCl | 125 mM | 3.653 g |
| Glucose | 10 mM | 901 mg |
| NaHCO3 | 26 mM | 1.092 g |
| NaH2PO4 | 1.25 mM | 75 mg |
| KCl | 2.5 mM | 93 mg |
| MgCl2·6H2O | 1 mM | 102 mg |
| CaCl2·2H2O | 2 mM | 147 mg |
| ddH2O | to 0.5 L |
pH is 7.3–7.4 when bubbled with 95% O2 and 5% CO2. Osmolarity is 300–310 mOsm/kg.
This solution may be used for up to 10 days after preparation if kept at 4–8 °C.
3. Potassium gluconate intracellular solution
| Reagent | Final concentration | Quantity |
|---|---|---|
| Potassium gluconate | 145 mM | 1.698 g |
| MgCl2·6H2O | 2.5 mM | 25.5 mg |
| Sodium ATP | 2 mM | 55 mg |
| Sodium GTP | 0.5 mM | 13 mg |
| HEPES | 10 mM | 119 mg |
| EGTA | 0.5 mM | 9.5 mg |
| ddH2O | to 50 mL |
Adjust pH to 7.3 with 1 M KOH solution. Osmolarity is 280–290 mOsm/kg.
Make 1 mL aliquots and freeze them at -20 °C.
Laboratory supplies
1. Dissection dish with Sylgard-lined bottom (Living Systems Instrumentation, catalog number: DD-90-S-BLK)
2. 25 G hypodermic needles (BD, catalog number: 300400)
3. 30 G hypodermic needles (BD, catalog number: 304000)
4. Super glue (cyanoacrylate, water-resistant gel)
5. Glass capillaries with filament O.D. 1.5 mm, I.D. 0.86 mm (e.g., Sutter Instruments, catalog number: BF-150-86-10; Harvard Apparatus, catalog number: GC150F-10)
6. Thin-walled glass capillaries without filament O.D. 1.5 mm, I.D. 1.17 mm (Warner Instruments, catalog number: G150T-3)
7. 1 mL Luer-Lok syringes (BD, catalog number: 309628)
8. 2.5 mL Luer-Lok syringes (BD, catalog number: 300185)
9. Silicon tubing (VWR, catalog number: 228-0701)
10. Three-way tap (BD Connecta, catalog number: 394601)
11. MicroFil pipette fillers (WPI, catalog number: MF28G67-5)
12. Sterile syringe filters 0.22 μm (Thermo Scientific, catalog number: 171-0020)
13. Glass capillaries for stereotaxic syringe (Sutter instruments, catalog number: BF100-50-10)
14. Surgical sutures or staples
15. Disposable laboratory gloves (any suitable ones)
16. Parafilm (Merck, catalog number: HS234526B)
17. Eye ointment (Vidisic, Bausch+Lomb)
18. 70% ethanol
19. Antiseptic solution (Betadine, 100 mg/mL, EGIS)
20. 1 mL insulin 29 G fixed needle syringes (BD Micro-Fine, catalog number: 324891)
21. Acrylic adhesive/dental cement (Stoelting, catalog number: 51459)
Equipment
Stereotaxic injections
1. Stereotaxic apparatus (David Kopf Instruments, model: 902 dual)
2. Syringe for stereotaxic injections (10 μL, 701 Hamilton syringe with removable needle, catalog number: 80314)
3. 1 mm Hamilton micropipette compression fitting set (Hamilton, catalog number: 55750-01RN)
4. Manual injector/micrometer (Starrett, model: 262M) or an automatic injection pump
5. Rodent warming system (Stoelting, model: 53800 series)
6. Fur clipper (Kent Scientific, model: CL7300-KIT)
7. Bead sterilizer (Merck, catalog number: Z378569)
8. Microdrill (Stoelting, product number: 58610V) and respective drill bits (preferably 0.6 mm, Stoelting, catalog number: 58609)
9. Optional: Rodent anesthesia system (VetEquip)
Dissection instruments
1. Dissection pad (could be made from Styrofoam tightly wrapped in foil)
2. Big scissors (F.S.T., catalog number: 14000-20)
3. Small scissors (F.S.T., catalog number: 14058-11)
4. Coarse forceps (F.S.T., catalog number: 11651-10)
5. Big spring scissors (F.S.T., catalog number: 15025-10)
6. Small spring scissors (F.S.T., catalog number: 15000-12)
7. Two curved forceps (F.S.T., catalog number: 11063-07)
8. Fine forceps (F.S.T., catalog number: 11413-11)
9. Scalpel blade #15 (F.S.T., catalog number: 10015-00) and appropriate scalpel handle (F.S.T., catalog number: 10003-12)
10. Small metal plate (preferably gold, D.IY., ~1.5 cm × 1 cm, should fit in experimental chamber)
Visualization and illumination
1. Stereomicroscope (Olympus, model: SZX7)
2. Light source (AmScope, model: 6 W LED Dual Gooseneck Illuminator)
3. Upright microscope (Olympus, model: BX50WI)
4. 60× water immersion objective (Olympus, model: LUMPlanFl 60×/0.90 W)
5. Low magnification (4–5×) objective (Carl Zeiss, model: Epiplan; Olympus, model Plan N)
6. Eyepiece micrometer (Evident, model: U-OCMC10/100X)
7. Infrared-sensitive CCD camera (Olympus, model: OLY-150IR or Dage-MTI, model: IR-2000)
8. Narrow beam (± 3°) infrared light emitting diode (IR-LED, Osram, models: SFH4550, SFH4545)
Critical: The spectral characteristics of the IR-LED and CCD camera should match.
9. White light–emitting diode (Dialight, model: 5219901802F)
10. Power supply unit (AimTTI, model: QL355T)
11. Monochromator (Till Photonics, model: Polychrome V)
Note: Blue LED laser (wavelength ~470 nm) may also be used for photostimulation.
12. Filter set (any suitable for ChR2 and EYFP excitation, e.g., Chroma Technology, model: 69008)
Perfusion
1. Gravity-fed perfusion system (any commercially available or self-made)
2. Peristaltic pump (Gilson, model: Minipuls 3)
3. Recording chamber (any that fits the microscope)
Electrophysiology
1. Patch clamp amplifier with respective headstage (Molecular Devices, model: Multiclamp 700B, headstage CV-7B)
2. Digitizer (Molecular Devices, model: Digidata 1440)
3. PC computer with Windows operating system
4. Patch clamp micromanipulator (Scientifica, model: PatchStar)
5. Constant current stimulator (A.M.P.I., model: ISO-FLEX)
6. Vibration isolation table (CleanBench, model: TMC)
7. Faraday cage (any commercially available or self-made)
8. Small three-axis micromanipulators (Narishige, model: UN-3C)
9. Pipette holders (Molecular devices, model: 1-HL-U)
10. Pipette puller (Sutter Instruments, model: P-97)
11. Silver wire AWG 26 for electrodes (WPI, product number: AGW1510)
12. Ag/AgCl pellets (WPI, product number: EP1)
13. BNC cables
14. Bunsen burner
Software and datasets
1. pClamp (version 10.7, Molecular Devices, July 2016)
2. Clampfit (version 10.7, Molecular Devices, July 2016)
3. TillVision (version 4.0, Till Photonics, 2008) or alternative laser controlling software
4. Origin (version 2022, OriginLab, June 2022)
5. MiniAnalysis (version 6.0.37, Synaptosoft Inc., 2009)
Procedure
文章信息
稿件历史记录
提交日期: Aug 18, 2025
接收日期: Sep 18, 2025
在线发布日期: Oct 10, 2025
出版日期: Nov 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Krotov, V., Blashchak, I., Moore, J., Moore, A., Romanenko, S., Voitenko, N. and Belan, P. (2025). Optogenetic Approach for Investigating Descending Control of Nociception in Ex Vivo Spinal Cord Preparation. Bio-protocol 15(21): e5483. DOI: 10.21769/BioProtoc.5483.
分类
神经科学 > 感觉和运动系统 > 脊髓
系统生物学 > 连接组学 > 细胞连接
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link