- EN - English
- CN - 中文
Rapid and Simplified Induction of Spinal Motor Neurons From Human Induced Pluripotent Stem Cells
从人诱导多能干细胞快速简便地诱导脊髓运动神经元
(*contributed equally to this work, § Technical contact) 发布: 2025年10月20日第15卷第20期 DOI: 10.21769/BioProtoc.5478 浏览次数: 1659
评审: Marion HoggAnonymous reviewer(s)

相关实验方案

人 iPSC 衍生神经元与少突胶质细胞共培养用于髓鞘形成的小分子筛选分析
Stefanie Elke Chie [...] Maria Consolata Miletta
2025年05月05日 3433 阅读
Abstract
Human induced pluripotent stem cell (hiPSC)-derived motor neurons (MNs) provide a critical source for the study of motor neuron diseases (MNDs), which has been hindered by the lack of appropriate disease models for many years. Although many spinal MN differentiation protocols have been established by mimicking in vivo neurogenesis using extrinsic signaling molecules, substantial variations in the duration and efficiency persist due to inconsistencies in concentrations, timing, and delivery methods of these molecules. Here, we present an efficient monolayer culture differentiation strategy that enables the generation of enriched CHAT+ spinal MNs (sMNs) in 18 days and functional sMNs exhibiting extensive network activities, as confirmed by multielectrode array (MEA), within 28 days. Therefore, this optimized MN differentiation protocol facilitates the production of mature sMNs for MND research, high-throughput drug screening, and potential cell replacement therapies.
Key features
• This protocol provides a rapid and simple monolayer culture strategy for differentiating hiPSCs into sMNs in 18 days.
• Early administration of the Notch inhibitor Compound E accelerates the generation of hiPSC–sMNs 10 days in advance compared to a previous protocol.
• This protocol uses neural stem cells (NSCs) and MNPs as an intermediate to generate functionally mature sMNs.
Keywords: hiPSC (人诱导多能干细胞(hiPSC))Graphical overview
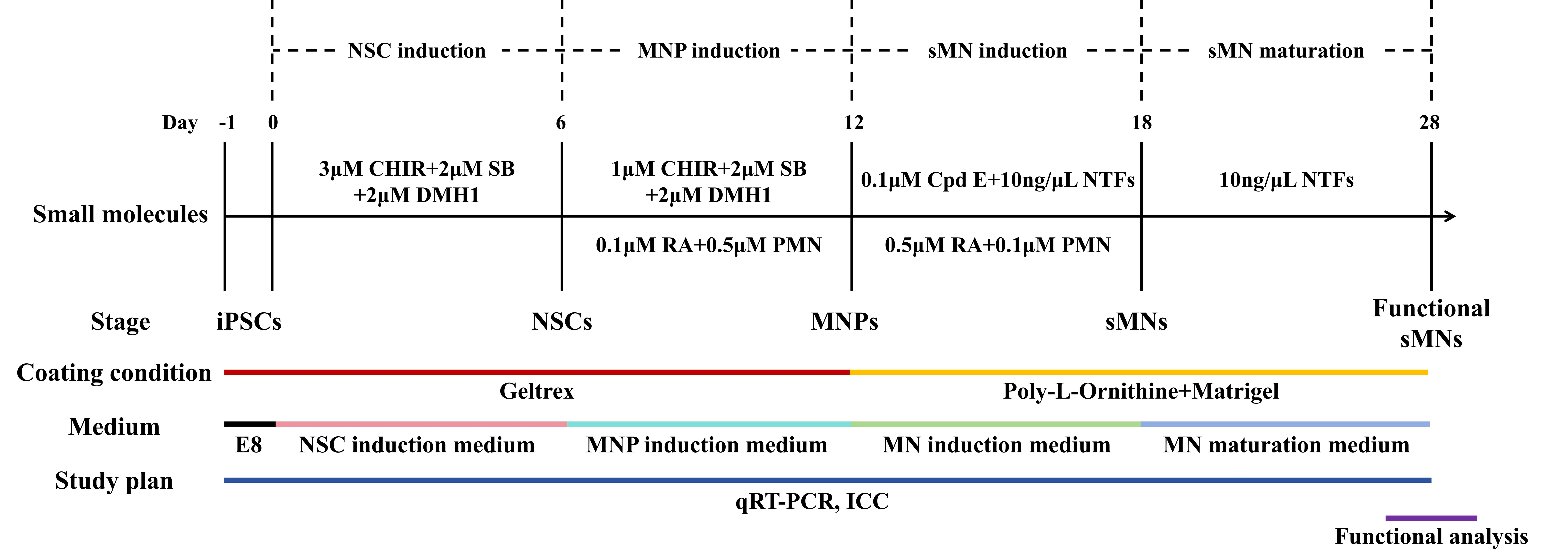
Schematic diagram of the spinal motor neuron (MN) differentiation protocol from iPSCs. CHIR, chir-99021; SB, SB-431542; RA, retinoic acid; PMN, purmorphamine; Cpd E, compound E; NTFs, neurotrophic factor.
Background
Spinal motor neurons (sMNs) are responsible for transmitting commands from the central nervous system (CNS) to muscles to control motor activity. sMNs are degenerated in motor neuron diseases (MNDs) such as amyotrophic lateral sclerosis (ALS), spinal muscular atrophy, and spinal and bulbar muscular atrophy. These MNDs are fatal diseases, and no effective treatment is available so far. This is thought to be caused, in part, by the lack of suitable human disease models [1,2]. The development of a robust protocol for the derivation of functional sMNs is, therefore, crucial for understanding disease mechanisms and developing novel therapies for MNDs.
As the signaling pathways and transcription factors contributing to sMN development have been unveiled [3–6], several protocols for sMN differentiation from pluripotent stem cells have been developed by mimicking in vivo neurogenesis using extrinsic signaling molecules [7,8]. Different doses, timing, and delivery procedures of signaling molecules among different protocols led to substantial variations in the duration (1–2 months) and efficiency (30%–90%) of MN differentiation [7–9]. In addition, the derived sMNs are often immature, and a lengthy maturation period is required before the detection of electrophysiological activity [10,11]. As most MNDs, including ALS, are late-onset diseases, these deficiencies limit the value of the derived sMNs in revealing disease characteristics.
By strategically advancing the temporal window for administering Compound E, a Notch signaling inhibitor, we have established a more efficient monolayer-based differentiation protocol compared to the previous method [12]. This precise timing intervention further enables the detection of extensive network activities in hiPSC–sMNs using a multielectrode array (MEA) as early as day 28 of differentiation. This novel MN differentiation protocol facilitates the production of mature sMNs for MND research, high-throughput drug screening, and potential cell transplantation therapy.
Materials and reagents
Biological materials
1. Human induced pluripotent stem cells (hiPSCs) (generated in-house [13])
Reagents
A. For cell culture
1. Essential 8TM Flex medium kit (E8 Flex medium) (Gibco, catalog number: A2858301)
2. GeltrexTM (Gibco, catalog number: A1413302)
3. KnockOutTM DMEM (Gibco, catalog number: 10829018)
4. Dulbecco’s phosphate-buffered saline, no calcium, no magnesium (DPBS w/o Ca2+/Mg2+) (Gibco, catalog number: 14190144)
5. Gentle cell dissociation reagent (Stem Cell Technologies, catalog number: 100-0485)
6. StemProTM AccutaseTM cell dissociation reagent (Thermo Fisher Scientific, catalog number: A1110501)
7. Knockout serum supplement (Gibco, catalog number: 10828028)
8. ROCK inhibitor Y-27632 (STEMCELL Technologies, catalog number: 72304)
9. DMEM/F12 with HEPES (Lonza, catalog number: LZBE12-719F)
10. Neurobasal medium (Gibco, catalog number: 21103049)
11. Penicillin/streptomycin (Gibco, catalog number: 15140122)
12. GlutaMAXTM supplement (Gibco, catalog number: 35050061)
13. N2 supplement (Gibco, catalog number: 17502048)
14. B27 supplement (Gibco, catalog number: 17504044)
15. L-ascorbic acid (Sigma, catalog number: A4544)
16. CHIR-99021 (MedChemExpress, catalog number: HY-10182)
17. SB-431542 (MedChemExpress, catalog number: HY-10431)
18. DMH-1 (MedChemExpress, catalog number: HY-12273)
19. Retinoic acid (MedChemExpress, catalog number: HY-14649)
20. Purmorphamine (MedChemExpress, catalog number: HY-15108)
21. Compound E (MedChemExpress, catalog number: HY-14176)
22. BDNF (PeproTech, catalog number: 450-02)
23. CNTF (PeproTech, catalog number: 450-13)
24. IGF-1 (PeproTech, catalog number: 100-11)
25. Poly-L-Ornithine (POL) (Sigma, catalog number: P4957)
26. Dulbecco’s phosphate-buffered saline (DPBS) (Sigma, catalog number: D8662)
27. Matrigel (Corning, catalog number: 354277)
28. Ethanol (Lennox, catalog number: 32221)
29. Dimethylsulfoxide (DMSO) (Sigma, catalog number: D2650)
30. Trypsin/EDTA (Gibco, catalog number: 25200056)
B. For immunocytochemistry (ICC)
1. Paraformaldehyde (PFA) (Santa Cruz, catalog number: 30525-89-4)
2. Bovine serum albumin (BSA) (Sigma, catalog number: A2153)
3. Phosphate-buffered saline (PBS) tablet (Fisher Scientific, catalog number: 12821680)
4. Triton X-100 (Sigma, catalog number: T8787)
5. Anti-NESTIN (Abcam, catalog number: ab22035)
6. Anti-PAX6 (Cell Signalling Technology, catalog number: 60433S, 1:500 dilution)
7. Anti-SOX2 (Cell Signalling Technology, catalog number: 3579, 1:500 dilution)
8. Anti-OCT4 (Cell Signalling Technology, catalog number: 2840, 1:500 dilution)
9. Anti-OLIG2 (Merck Millipore, catalog number: AB9610, 1:500 dilution)
10. Anti-NKX6.1 (DSHB, catalog number: F55A10-s, 1:50 dilution)
11. Anti-ISL1 (Sigma, catalog number: HPA057416, 1:500 dilution)
12. Anti-MNX1 (DSHB, catalog number: 81.5C10-s, 1:20 dilution)
13. Anti-CHAT (Merck Millipore, catalog number: AB144P, 1:100 dilution)
14. Anti-MAP2 (Proteintech, catalog number: 17490-1-AP, 1:500 dilution)
15. Anti-mouse IgG (H+L), F(ab’)2 fragment (Alexa Fluor® 488 conjugate) (Cell Signalling Technology, catalog number: 4408s, 1:1,000 dilution)
16. Anti-rabbit IgG (H+L), F(ab’)2 fragment (Alexa Fluor® 555 conjugate) (Cell Signalling Technology, catalog number: 4413s, 1:1,000 dilution)
17. Donkey anti-goat IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor 488 (Millipore, catalog number: A-11055, 1:1,000 dilution)
18. Donkey anti-rabbit IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor 647 (Millipore, catalog number: A-31573, 1:1,000 dilution)
19. Hoechst 33342 (Invitrogen, catalog number: H3569, 1:2,000 dilution)
Note: All reagents should be stored following the manufacturer's instructions.
Solutions
1. Complete E8 Flex medium for iPSC culture (see Recipes)
2. Geltrex solution for coating (see Recipes)
3. Basal medium for MN differentiation (see Recipes)
4. NSC induction medium (see Recipes)
5. MNP induction medium (see Recipes)
6. MN induction medium (see Recipes)
7. MN maturation medium (see Recipes)
8. Matrigel solution for coating (see Recipes)
9. Stock solutions of small molecules/growth factors for MN differentiation:
a. 100 mM L-ascorbic acid (see Recipes)
b. 10 mM CHIR99021 (see Recipes)
c. 10 mM DMH1 (see Recipes)
d. 10 mM SB431542 (see Recipes)
e. 10 mM retinoic acid (see Recipes)
f. 10 mM Purmorphamine (see Recipes)
g. Compound E (see Recipes)
h. 100 μg/mL BDNF (see Recipes)
i. 100 μg/mL CNTF (see Recipes)
j. 100 μg/mL IGF-1 (see Recipes)
10. 0.1% BSA in PBS (see Recipes)
11. 1× PBS solution (see Recipes)
12. 70% and 90% ethanol (see Recipes)
13. 1% BSA + 0.3% Triton X-100 in PBS (see Recipes)
Recipes
1. Complete E8 Flex medium for hiPSCs
a. Thaw the frozen E8 Flex supplement at 2–8 °C overnight. Protect the supplement from light, as it is light-sensitive.
b. Mix the thawed supplement by gently inverting the vial several times, then aseptically transfer all contents to the bottle of E8 Flex basal medium. Swirl the bottle to mix.
c. Complete E8 Flex medium can be stored at 2–8 °C for up to 2 weeks. Alternatively, aliquot 45 mL of complete E8 Flex medium into 50 mL Falcon tubes and store at -20 °C for up to 6 months. Avoid repeated freeze-thaw cycles.
d. Before use, warm the complete E8 Flex medium at room temperature and protect it from light.
Critical: Do not warm the medium in a 37 °C water bath.
2. Geltrex solution for coating
a) Geltrex stock solution (1:1 dilution)
i. Thaw the frozen Geltrex overnight at 2–8 °C.
ii. Mix 5 mL of Geltrex with 5 mL of pre-chilled KnockOutTM DMEM/F12.
iii. Aliquot Geltrex stock solution (1:1 dilution) into a 1 mL cryogenic tube and store at -80 °C for several months.
b) Geltrex working solution (1:100 dilution)
i. Thaw 1 mL of frozen Geltrex stock solution at 2–8 °C overnight.
ii. Dilute 1 mL of Geltrex stock solution into 49 mL of pre-chilled KnockOutTM DMEM/F12 to get a 1:100 diluted Geltrex working solution.
iii. The working solution can be stored at 2–8 °C for up to 2 weeks.
3. Basal medium for MN differentiation
| Reagent | Final concentration | Volume |
| DMEM/F12 with HEPES | 49% | 245 mL |
| Neurobasal medium (0.5× volume) | 49% | 245 mL |
| 1× Penicillin/streptomycin | 1% | 5 mL |
| 1× GlutaMAXTM supplement | 1% | 5 mL |
| Filter the above components through a 0.22 μm sterile filter | ||
| 0.5× N2 supplement | 2.5 mL | |
| 0.5× B27 supplement | 5 mL | |
The basal medium can be stored at 2–8 °C for up to 4 weeks. Alternatively, aliquot 45 mL of basal medium into 50 mL Falcon tubes and store at -20 °C for up to 6 months.
Note: Protect the medium from light, as N2 and B27 are light-sensitive. Avoid repeated freeze-thaw cycles.
4. NSC induction medium
| Reagent | Final concentration | Volume |
|---|---|---|
| Basal medium | ~100% | accordingly |
| L-ascorbic acid | 0.1 mM | see Recipe 9a |
| CHIR99021 | 3 μM | see Recipe 9b |
| DMH1 | 2 μM | see Recipe 9c |
| SB431542 | 2 μM | see Recipe 9d |
Before use, warm the basal medium at room temperature and protect it from light. Do not warm the medium in a 37 °C water bath. To prepare the NSC induction medium, first aliquot the basal medium according to the volume required. Add small molecules to the medium in the amounts specified in the table.
5. MNP induction medium
| Reagent | Final concentration | Volume |
|---|---|---|
| Basal medium | ~100% | accordingly |
| L-ascorbic acid | 0.1 mM | see Recipe 9a |
| Retinoic acid | 0.1 μM | see Recipe 9e |
| Purmorphamine | 0.5 μM | see Recipe 9f |
| CHIR99021 | 1 μM | see Recipe 9b |
| DMH1 | 2 μM | see Recipe 9c |
| SB431542 | 2 μM | see Recipe 9d |
Before use, warm the basal medium at room temperature and protect it from light. Do not warm the medium in a 37 °C water bath. To prepare the MNP induction medium, first aliquot the basal medium according to the volume required. Add small molecules to the medium in the amounts specified in the table.
6. MN induction medium
| Reagent | Final concentration | Volume |
|---|---|---|
| Basal medium | ~100% | accordingly |
| L-ascorbic acid | 0.1 mM | see Recipe 9a |
| Retinoic acid | 0.5 μM | see Recipe 9e |
| Purmorphamine | 0.1 μM | see Recipe 9f |
| Compound E | 0.1 μM | see Recipe 9g |
| BDNF | 10 ng/μL | see Recipe 9h |
| CNTF | 10 ng/μL | see Recipe 9i |
| IGF-1 | 10 ng/μL | see Recipe 9j |
Before use, warm the basal medium at room temperature and protect it from light. Do not warm the medium in a 37 °C water bath. To prepare the MN induction medium, first aliquot the basal medium according to the volume required. Add small molecules to the medium in the amounts specified in the table.
7. MN maturation medium
| Reagent | Final concentration | Volume |
|---|---|---|
| Basal medium | ~100% | accordingly |
| L-ascorbic acid | 0.1 mM | see Recipe 9a |
| BDNF | 10 ng/μL | see Recipe 9h |
| CNTF | 10 ng/μL | see Recipe 9i |
| IGF-1 | 10 ng/μL | see Recipe 9j |
Before use, warm the basal medium at room temperature and protect it from light. Do not warm the medium in a 37 °C water bath. To prepare MN maturation medium, first aliquot the basal medium according to the volume required. Add neurotrophic factors (NTFs) to the medium in the amounts specified in the table.
8. Matrigel solution for coating
a) Matrigel stock solution (1:1 dilution)
i. Thaw the frozen Matrigel at 2–8 °C overnight.
ii. Mix 5 mL of Matrigel with 5 mL of pre-chilled KnockOutTM DMEM/F12.
iii. Aliquot Matrigel stock solution (1:1 dilution) into a 1 mL cryogenic tube and store at -80 °C for up to one year.
b) Matrigel working solution (1:50 dilution)
i. Thaw 1 mL of frozen Matrigel stock solution at 2–8 °C overnight.
ii. Dilute 1 mL of Matrigel stock solution in 24 mL of pre-chilled KnockOutTM DMEM/F12 to obtain a 1:50 diluted Matrigel working solution.
iii. The working solution can be stored at 2–8 °C for up to 2 weeks.
Note: Avoid repeated freeze-thaw cycles of the working Matrigel solution.
9. Stock solutions of small molecules/growth factors for MN differentiation
Notes:
1. Information regarding the stability of small molecules in solution is rarely reported. The recommended storage time for each small molecule after preparation should follow the supplier’s guidelines.
2. Protect all small molecules from light.
a. 100 mM L-ascorbic acid
Dissolve 88.06 mg of L-ascorbic acid in 5 mL of sterile MilliQ water. Filter the solution through a 0.22 μm sterile filter. Aliquot 50 μL per tube and store at -80 °C for up to two years.
b. 10 mM CHIR99021
Dissolve 5 mg of CHIR99021 in 1.0745 mL of DMSO. Aliquot 10 μL per tube and store at -80 °C for up to one year.
c. 10 mM DMH1
Dissolve 10 mg of DMH1 in 2.6285 mL of DMSO. Aliquot 10 μL per tube and store at -80 °C for two years.
d. 10 mM SB431542
Dissolve 10 mg of SB431542 in 2.6012 mL of DMSO. Aliquot 10 μL per tube and store at -80 °C for two years.
e. 10 mM retinoic acid
Dissolve 100 mg of retinoic acid in 3.3285 mL of DMSO to prepare a 100 mM stock solution. Further dilute to 10 mM as needed. Aliquot 10 μL per tube and store at -80 °C for 6 months.
f. 10 mM Purmorphamine
Dissolve 5 mg of Purmorphamine in 960.393 μL of DMSO. Aliquot 10 μL per tube and store at -80 °C for up to two years.
g. Compound E
Dissolve 1 mg of Compound E in 203.874 μL of DMSO. Aliquot 10 μL per tube and store at -80 °C for up to 6 months.
h. 100 μg/mL BDNF
Dissolve 100 μg of BDNF in 1 mL of sterile 0.1% BSA in PBS. Aliquot 20 μL per tube and store at -80 °C for up to one year.
i. 100 μg/mL CNTF
Dissolve 100 μg of CNTF in 1 mL of sterile 0.1% BSA in PBS. Aliquot 20 μL per tube and store at -80 °C for up to one year.
j. 100 μg/mL IGF-1
Dissolve 100 μg IGF-1 in 1 mL of sterile 0.1% BSA in PBS. Aliquot 20 μL per tube and store at -80 °C for up to one year.
10. 0.1% BSA in PBS
Dissolve 0.1 g of BSA in 100 mL of sterile DPBS solution. Filter the solution through a 0.22 μm sterile filter. Aliquot 5 mL per tube and store at -20 °C for up to one year.
11. 1× PBS solution
Dissolve one PBS tablet in 100 mL of distilled water using a magnetic stirrer and stir bar. Store at room temperature for up to one month.
12. 70% and 90% ethanol
To prepare 500 mL of 70% ethanol, combine 350 mL of 100% ethanol with 150 mL of distilled water. To prepare 500 mL of 90% ethanol, combine 450 mL of 100% ethanol with 50 mL of distilled water.
13. 1% BSA + 0.3% Triton X-100 in PBS
Dissolve 0.1 g of BSA in 10 mL of 1× PBS solution by vortexing. Once fully dissolved, add 30 μL of Triton X-100 to the solution and vortex thoroughly to mix. Store at 4 °C for up to one month.
Laboratory supplies
1. 6-well tissue culture plate (Sarstedt, catalog number: 83.3920.300)
2. Centrifuge tubes, 15 and 50 mL (Sarstedt, catalog numbers: 62.554.502 and 62.547.254)
3. Microcentrifuge tube, 1.5 mL (Sarstedt, catalog number: 72.690.001)
4. Cell scraper (Sarstedt, catalog number: 83.1831)
5. μ-slide 8 well (ibidi Gmbh, catalog number: 80826)
6. 0.22 μm disposable sterile filters (Lennox, catalog number: A16534K)
7. 1.5 mL microcentrifuge tube (Sarstedt, catalog number: 72.690.001)
8. Filter tip 100–1,000 μL (Cruinn, catalog number: S1122-1830)
9. Filter tip 0.1–10 μL (Cruinn, catalog number: S1121-3810)
Equipment
1. CO2 incubator (Thermo Fisher Scientific, model: 371)
2. Stereo microscope (Thermo Fisher, model: EVOS XL Core)
3. Inverted light microscope (Zeiss, model: Axiovert 40 CFL)
4. Confocal microscope FV1000 (Olympus)
5. Maestro Original (Axion BioSystems)
6. CytoView MEA 48 plate (Axion BioSystems)
7. Water bath (Fisher Scientific, model: FSGPD28)
8. Operetta Imaging system with Harmony image analysis software (Perkin Elmer)
Software and datasets
1. AxIS Navigator, CiPA Analysis Tool: https://www.axionbiosystems.com/products/mea/mea-software
2. Fiji ImageJ-win64
Procedure
文章信息
稿件历史记录
提交日期: Jul 21, 2025
接收日期: Sep 9, 2025
在线发布日期: Sep 23, 2025
出版日期: Oct 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Chen, Y., Yang, F., O'Brien, T., Shen, S. and Yang, M. (2025). Rapid and Simplified Induction of Spinal Motor Neurons From Human Induced Pluripotent Stem Cells. Bio-protocol 15(20): e5478. DOI: 10.21769/BioProtoc.5478.
分类
干细胞 > 多能干细胞 > 细胞分化
神经科学 > 神经系统疾病 > 细胞机制
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link