- EN - English
- CN - 中文
Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
由香蕉 (Musa spp.)未成熟雄花序直接再生植株
发布: 2025年10月20日第15卷第20期 DOI: 10.21769/BioProtoc.5476 浏览次数: 1438
评审: Diarmuid Seosamh Ó’MaoiléidighAnonymous reviewer(s)
Abstract
Banana (Musa spp.) is a globally important horticultural crop that faces significant challenges from pests and diseases, which threaten yield and long-term sustainability. The efficient production of clean, disease-free planting material is essential for both commercial plantations and small-holder systems. This paper presents a rapid and reproducible protocol for direct plant regeneration from immature male inflorescences of banana. The method involves surface sterilization of immature male flowers, longitudinal dissection, and culture on Murashige and Skoog (MS) medium supplemented with 6-benzylaminopurine (BAP), enabling direct shoot regeneration from floral meristems without an intermediate regenerable callus phase. This approach offers several advantages over traditional embryogenic cell suspension (ECS) methods, including simplified sterilization, high regeneration efficiency, and scalability. The protocol was successfully applied to multiple banana cultivars, including Cavendish (AAA) and Lady Finger (AAB), achieving 100% shoot regeneration efficiency with plantlet production within 6–8 months. This protocol provides a reliable and efficient alternative for rapid mass propagation of banana plants, supporting sustainable production and research applications.
Key features
• The protocol can be performed in a standard tissue culture lab without expensive instruments or complex setup, making it accessible for labs in resource-limited settings.
• Minimal contamination risk since immature male inflorescences enclosed within bracts are naturally protected, and the simplified sterilization procedure leads to consistently low contamination rates.
• Potential for high multiplication where each immature male flower produces 50–100 shoots under optimized conditions, reducing the number of subcultures needed for large-scale propagation.
• The method performed equally well in genetically distinct banana cultivars (AAA and AAB groups), suggesting broader applicability across diverse Musa genotypes.
Keywords: Musa spp. (Musa spp.)Graphical overview
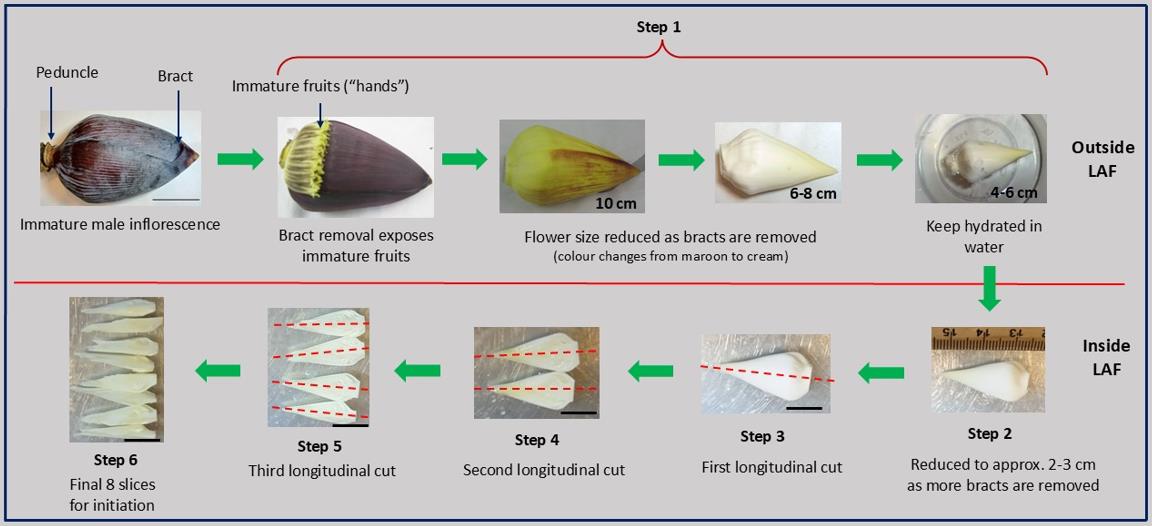
Stepwise longitudinal dissection of the banana bell for tissue culture. Bracts (maroon sheaths) are sequentially peeled until a cream-colored flower (~4–6 cm) is exposed and kept hydrated in sterile water in a small container (step 1). The bell is further reduced to approximately 2–3 cm under sterile conditions in a laminar air flow (LAF) hood (step 2), followed by a longitudinal cut along the red dashed line (step 3). Two subsequent longitudinal cuts (steps 4 and 5) produce eight thin slices (step 6): first cut → 2 slices, second → 4 slices, third → 8 slices. These final eight slices are used for culture. Scale bars: 5 cm (top panel) and 2 cm (bottom panel). Figure created by the author using original photographs of banana flowers taken by the author. All arrows, lines, and labels were added using Microsoft PowerPoint.
Background
Banana (Musa spp.) is a versatile horticultural crop grown globally for its sweet fruit, typically consumed fresh. In regions such as East Africa, bananas are also a staple when consumed for cooking as green bananas [1]. In addition to its subsistence role, the banana is a major commercial commodity with significant economic value in global markets [2,3].
Despite its importance, banana production is severely threatened by pests and diseases, including Fusarium wilt (Panama disease), Black Sigatoka, and Banana Bunchy Top Virus (BBTV), which collectively reduce yield and compromise crop sustainability [4,5]. As a result, there is an increasing demand for clean, disease-free planting materials to support both commercial plantations and smallholder farming systems [6].
Tissue culture has emerged as a vital tool for the rapid, large-scale production of uniform and disease-free banana plants. Currently, three main methodologies are used for banana micropropagation. The method of somatic embryogenesis via embryogenic cell suspension (ECS) begins with the induction of embryogenic calli from immature male inflorescences cultured on Murashige and Skoog (MS) medium [7] supplemented with plant growth regulators. These calli are transferred to liquid medium to establish ECS, which are later plated to develop somatic embryos. However, the efficiency of embryogenic callus induction is typically very low (~5%), and obtaining such calli from any single inflorescence is rare. In practice, multiple inflorescences must be dissected per cultivar to establish ECS cultures. An alternative ECS approach involves scalping, wherein in vitro plants are maintained on 6-Benzylaminopurine (BAP)-enriched media for 12–18 months to generate dense meristematic clumps (scalps) that are induced to form embryogenic calli [8] and references therein. However, this method is highly time-consuming, genotype-dependent, and resource-intensive.
The method of meristem culture from field-grown suckers or offshoots uses vegetative buds from suckers as explants. However, extensive surface sterilization is required, and initial cultures are often plagued by contamination. While shoot proliferation can be achieved using high levels of BAP and adenine hemisulfate, establishing contamination-free initial cultures remains a major bottleneck [9].
The method proposed here, direct regeneration from immature male inflorescence (our proposed method), starts with immature male flowers (commonly referred to as “bells”) being surface-sterilized using 70% ethanol, rinsed with sterile water, trimmed to approximately 4 cm, and longitudinally sectioned. The explants are cultured on MS medium supplemented with BAP, enabling floral meristems to directly reprogram into shoot meristems—bypassing the callus stage entirely. Generally, in most regeneration systems, when explants are placed on an appropriate medium containing an auxin and a cytokinin, the explants undergo dedifferentiation and form callus, which is a mass of undifferentiated cells. These calli are then reprogrammed by altering the concentrations of the hormones and media components to differentiate into a shoot and ultimately into a plantlet. However, in this direct regeneration, there is no formation of a regenerable calli. The shoots are formed by reprogramming the floral meristems into a shoot meristem.
This method offers several key advantages over existing protocols, namely simplified sterilization, as the enclosed nature of immature inflorescences minimizes contamination risk, requiring only basic surface sterilization procedures; high responsiveness, as nearly every flower can regenerate shoots, offering a major advantage over the low-efficiency ECS method; high yield per explant, since each flower has the capacity to produce large numbers of clonal plantlets, with reports of up to 80–130 under optimized conditions in other cultivars [10], reducing the number of subculture cycles required; and a scalable and genotype-flexible, as this method is suitable for both commercial-scale production and research, and has shown consistent performance across multiple Musa genotypes.
While similar methods have been briefly reported [10–12], these protocols lack sufficient detail for reliable replication. This method has been successfully developed and tested using Musa cultivars Cavendish (AAA) and Lady Finger (AAB), consistently achieving 100% shoot regeneration efficiency. The method was later adopted by others, who also achieved similar results—demonstrating its robustness and reproducibility.
In addition to micropropagation, this protocol may have broader applications in areas such as in vitro mutagenesis, transgenic line development, or virus indexing, where efficient plant regeneration is required from floral tissues.
Materials and reagents
Biological materials
1. Immature male inflorescences at least 10 from field plants
Reagents
1. Murashige and Skoog (MS) basal medium (Sigma, catalog number: M5524; contains MS macro and micro salts without vitamins)
2. Murashige and Skoog (MS) basal medium (Sigma, catalog number: M5519; contains MS macro, micro, and vitamins)
3. Biotin powder (Austratec, catalog number: B140)
4. 6-Benzylaminopurine (BAP) powder (Austratec, catalog number: B800)
5. 1-Naphthaleneacetic acid (NAA) powder (Austratec, catalog number: N600)
6. Indole-3-acetic acid (IAA) powder (Austratec, catalog number: I885)
7. Morel & Wetmore vitamin solution (100×) (Austratec, catalog number: M592)
8. Sucrose (PhytoTech Labs, catalog number: S391)
9. Phytagel (Sigma, catalog number: P8169)
10. Ethanol (70% and absolute) (Sigma, catalog number: 459844)
11. Sterile distilled or Milli-Q water
12. 1 M NaOH (Sigma, catalog number: 1091371000)
Solutions
1. Biotin stock (1 mg/mL) (see Recipes)
2. BAP stock (1 mg/mL) (see Recipes)
3. NAA stock (1 mg/mL) (see Recipes)
4. IAA stock (1 mg/mL) (see Recipes)
5. 2× Meristem formation media (see Recipes)
6. 2× Meristem proliferation media (see Recipes)
7. 2× Shoot differentiation media (see Recipes)
8. 2× Plant rooting and development media (see Recipes)
Recipes
1. Biotin stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Biotin | 1 mg/mL | 20 mg |
| Distilled water | To 20 mL | |
| Total | 20 mL |
Note: Biotin is only sparingly soluble in cold water. Warm the water to 40–50 °C before adding biotin to aid dissolution. Do not boil. Filter-sterilize the solution using a 0.22 μm filter. Dispense 1,000 μL aliquots into sterile 1.5 mL microfuge tubes. Store at -20 °C.
2. BAP stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BAP (6-Benzylaminopurine) | 1 mg/mL | 20 mg |
| 1 M NaOH | 100 μL for dissolution | |
| Distilled water | To 20 mL | |
| Total | n/a | 20 mL |
Note: Dissolve BAP in a few drops of 1 N NaOH or ethanol before adding distilled water to make up 20 mL. Filter-sterilize using a 0.22 μm filter. Dispense 1,000 μL aliquots into sterile 1.5 mL microfuge tubes. Store at -20 °C.
3. NAA stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NAA (naphthaleneacetic acid) | 1 mg/mL | 20 mg |
| Absolute ethanol (100%) | 14 mL | |
| Distilled water | To 20 mL (6 mL) | |
| Total | n/a | 20 mL |
Note: Dissolve NAA in 14 mL of absolute ethanol, then add 6 mL of distilled water to reach 20 mL (final ethanol concentration: 70%). Filter sterilize (0.22 μm), aliquot at 1,000 μL, and store at -20 °C.
4. IAA stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| IAA (Indole-3-acetic acid) | 1 mg/mL | 20 mg |
| Absolute ethanol (100%) | 14 mL | |
| Distilled water | To 20 mL (6 mL) | |
| Total | n/a | 20 mL |
Note: Dissolve IAA in 14 mL of absolute ethanol, then add 6 mL of distilled water to reach 20 mL (final ethanol concentration: 70%). Filter sterilize (0.22 μm), protect from light, aliquot at 1,000 μL, and store at -20 °C.
5. 2× Meristem formation media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5519) | 4.43 g/L | 4.43 g |
| Sucrose | 30 g/L | 30 g |
| Biotin stock (1 mg/mL) | 1 mg/L | 1 mL |
| BAP stock (1 mg/mL) | 5 mg/L and 7 mg/L | 5 mL and 7 mL (two batches) |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Note: When working with a new cultivar, it is recommended to try both 5 and 7 mg/L BAP concentrations to determine which is more effective for initiating meristem formation. Cytokinin responsiveness can vary between genotypes.
a. Dissolve MS powder (M5519) in 700 mL of sterile distilled water.
b. Add sucrose and mix well.
c. Add 1 mL of biotin (1 mg/mL stock) to achieve 1 mg/L final concentration.
d. Prepare two separate batches of media:
i. Add 5 mL of BAP stock (1 mg/mL) to one (for a 5 mg/L final concentration).
ii. Add 7 mL of BAP stock (1 mg/mL) to the second (for a 7 mg/L final concentration).
e. Adjust pH to 5.7 using 1 M NaOH (or HCl if needed).
f. Make up the volume to 1 L with distilled water.
g. Add 3 g of phytagel.
h. Autoclave at 121 °C, 15 psi for 15 min.
i. Cool to 50–60 °C and pour 25–30 mL per sterile 90 mm deep Petri plate under laminar airflow.
6. 2× Meristem proliferation media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5519) | 4.43 g/L | 4.43 g |
| Sucrose | 30 g/L | 30 g |
| Biotin stock (1 mg/mL) | 1 mg/L | 1 mL |
| NAA stock (1 mg/mL) | 1 mg/L | 1 mg |
| BAP stock (1mg/mL) | 5 mg/L and 7 mg/L | 5 mL and 7 mL (two batches) |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Note: As with meristem formation, when working with a new cultivar, it is recommended to try both 5 and 7 mg/L BAP concentrations, now in combination with 1 mg/L NAA, to determine the optimal hormonal balance for meristem proliferation. Auxin–cytokinin synergy can significantly influence shoot multiplication rates.
a. Dissolve MS powder (M5519) in ~700 mL of sterile distilled water.
b. Add sucrose and mix well.
c. Add 1 mL of biotin stock (1 mg/mL).
d. Prepare two separate media batches:
e. Add 5 mL of BAP stock (1 mg/mL) to one batch (for 5 mg/L BAP).
f. Add 7 mL of BAP stock (1 mg/mL) to the second batch (for 7 mg/L BAP).
g. Add 1 mL of NAA stock (1 mg/mL) to each batch.
h. Adjust pH to 5.8 using 1 M NaOH.
i. Bring volume to 1,000 mL with distilled water.
j. Add 3 g of phytagel per liter.
k. Autoclave at 121 °C, 15 psi, for 15 min.
l. Cool to 50–60 °C and pour 25–30 mL per sterile 90 mm deep Petri plate under laminar airflow.
7. 2× Shoot differentiation media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5519) | 4.43 g/L | 4.43 g |
| Sucrose | 30 g/L | 30 g |
| Biotin stock (1 mg/mL) | 1 mg/L | 1 mL |
| BAP stock (1mg/mL) | 2 mg/L | 2 mL |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Notes:
1. After successful meristem initiation and proliferation, the concentration of BAP is reduced from 5 or 7 mg/L to 2 mg/L in the shoot differentiation stage to encourage organized shoot development rather than excessive callus or shoot clustering.
2. High cytokinin levels (like 5–7 mg/L BAP) are beneficial for stimulating shoot bud formation, but prolonged exposure may inhibit elongation or result in vitrification. Reducing BAP supports shoot elongation and differentiation into distinct, transplantable shoots, particularly important for downstream rooting and acclimatization stages.
a. Dissolve 4.43 g of MS powder (M5519) in approximately 700 mL of sterile distilled water.
b. Add 30 g of sucrose and stir until fully dissolved.
c. Add 1 mL of biotin stock (1 mg/mL) to achieve a final concentration of 1 mg/L.
d. Add 2 mL of BAP stock (1 mg/mL) to reach a final concentration of 2 mg/L.
e. Adjust the pH of the solution to 5.8 using 1 M NaOH (or HCl if needed).
f. Make up the final volume to 1,000 mL with sterile distilled water.
g. Add 3 g of phytagel and mix well.
h. Autoclave the media at 121 °C, 15 psi, for 15 min.
i. Allow to cool to 50–60 °C.
j. Pour 25–30 mL of the media into sterile 90 mm deep Petri plates under laminar airflow.
8. 2× Plant rooting and development media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5524) | 4.43 g/L | 4.43 g |
| Morel & Wetmore vitamin | 1× | 10 mL |
| Sucrose | 30 g/L | 30 g |
| BAP stock (1 mg/mL) | 0.045 mg/L | 45 μL |
| IAA stock (1 mg/mL) | 0.2 mg/L | 200 μL |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Notes:
1. Root induction requires a hormonal environment with minimal cytokinin and low auxin, mimicking the natural physiological conditions needed for root initiation.
2. In this medium, BAP is reduced to 0.045 mg/L, and IAA is included at 0.2 mg/L, providing a hormonal balance conducive to healthy root development without excessive callusing or shoot regeneration.
3. The inclusion of Morel & Wetmore vitamins supports root formation by supplying essential cofactors. Notably, this mix contains calcium pantothenate (Vitamin B5), which enhances root development by promoting coenzyme A biosynthesis—a vital pathway for energy metabolism and cell division in developing root tissues.
4. Since IAA (Indole-3-acetic acid) is heat labile, it should indeed be added after autoclaving, once the medium has cooled to around 60 °C under sterile conditions. This helps preserve its biological activity and ensures consistent rooting results.
a. Dissolve MS powder and sucrose in approximately 700 mL of distilled water.
b. Add Morel & Wetmore Vitamin solution and BAP stock. Mix thoroughly.
c. Adjust the pH to 5.8 using 1 M NaOH or HCl.
d. Bring the volume up to 1,000 mL with distilled water.
e. Add phytagel and stir to disperse evenly.
f. Autoclave the media at 121 °C, 15 psi, for 15 min.
g. Allow the media to cool to 50–60 °C.
h. Under sterile conditions in a laminar airflow hood, add IAA stock (200 μL of 1 mg/mL) to the cooled media.
i. Mix gently and pour 70 mL per sterile 250 mL culture container under laminar airflow.
Laboratory supplies
1. Beakers (250 mL, 500 mL, 1 L)
2. Measuring cylinders (10 mL to 1 L)
3. Magnetic stirrer and stir bars (or glass rod for manual stirring)
4. Pipettes (adjustable micropipettes: 10–1,000 μL) and sterile tips
5. Syringe and 0.22 μm syringe filters (for sterilizing hormone stock solutions)
6. Sterile 250 mL culture containers or jars (with lids or breathable covers)
7. Forceps and scalpels (sterile, for explant transfer)
8. Glass or plastic media bottles (autoclavable, 1 L capacity)
9. Parafilm or sealing tape (for sealing culture vessels)
10. Lab marker pens (for labeling media and containers)
11. Autoclave-safe gloves and lab coat
12. Waste containers for biological and chemical waste
Equipment
1. Analytical balance for accurate weighing of media components
2. pH meter for adjusting medium pH
3. Autoclave for sterilizing media and reusable supplies
4. Laminar airflow hood (LAF cabinet) for aseptic operations
5. Hot plate with magnetic stirrer for dissolving components and mixing medium
6. Refrigerator/freezer (-20 °C) for storing stock solutions
7. Growth chamber or culture room for incubating cultures under controlled temperature and photoperiod
8. Water bath (optional) for gently warming stock solutions
9. Distilled or MilliQ water system for preparing media
10. Light meter (lux or PAR) (optional) to monitor light levels in culture room
Procedure
文章信息
稿件历史记录
提交日期: Jun 27, 2025
接收日期: Sep 9, 2025
在线发布日期: Sep 23, 2025
出版日期: Oct 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Deo, P. C. (2025). Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.). Bio-protocol 15(20): e5476. DOI: 10.21769/BioProtoc.5476.
分类
植物科学 > 植物育种 > 微体繁殖
植物科学 > 植物发育生物学 > 形态建成
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












