- EN - English
- CN - 中文
High-Dimensional Phospho-CyTOF Characterization of T-Cell Activation Responses in Whole Blood
利用高维磷酸化CyTOF表征全血中T细胞的激活反应
(§Technical contact: Ilyssa.Ramos@nih.gov) 发布: 2025年10月20日第15卷第20期 DOI: 10.21769/BioProtoc.5473 浏览次数: 2694
评审: Samik BhattacharyaAnonymous reviewer(s)

相关实验方案

基于 T 细胞的平台,用于对肿瘤浸润 T 细胞的单细胞 RNA 测序数据集中鉴定的 T 细胞受体进行功能筛选
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
2024年04月20日 6409 阅读

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3550 阅读
Abstract
Recent advances in single-cell technologies have provided limited insight into the role of protein phosphorylation in T-cell fate and function. Dysregulated protein phosphorylation is associated with adverse clinical outcomes, emphasizing the need for reliable methods to unravel the complexities of T-cell signal transduction and disease-related alterations. While flow cytometry is widely used, it is constrained by spectral overlap, limiting the number of protein targets for simultaneous analysis. To overcome this, we present a robust protocol for whole blood T-cell stimulation and subsequent analysis using mass cytometry by time-of-flight (CyTOF). CyTOF minimizes spillover into adjacent channels by employing highly pure, stable, heavy metal–conjugated antibodies for protein detection. This protocol offers a high-dimensional approach for phenotypic and phospho-protein characterization of key signaling pathways, including JAK/STAT, MAPK, PI3K/mTOR, PKC, and NF-κB. A key feature is the T-cell stimulation reagent, which mimics endogenous activation by engaging the T-cell receptor (TCR)/CD3 complex and providing co-stimulation via an anti-CD28 antibody. Further, we enhance reproducibility and enable batch processing through the implementation of the Prot1/Thaw-Lyse system for immediate cryopreservation of stimulated blood samples. By employing CyTOF, this method permits the simultaneous analysis of 31 protein targets with single-cell resolution, minimizing spillover and providing superior specificity, sensitivity, and resolution over flow cytometric methods. This approach facilitates the robust assessment of TCR activation and its effect on bystander populations, which has been challenging with spectral flow cytometry due to the limited availability of methanol-resistant fluorophores. This protocol is a precise and reproducible method for elucidating the downstream effects of T-cell stimulation and immune status, with significant potential for clinical applications, including the assessment of T-cell-targeted therapies.
Key features
• Mass cytometry (CyTOF) analysis: A "vein-to-tube" protocol for high-dimensional profiling of key signaling pathways (JAK/STAT, MAPK, PI3K/mTOR, PKC, NF-κB) in human whole blood.
• Cryopreservation for batch processing: Enables long-term storage and standardized batch processing of whole blood samples by implementing immediate cryopreservation via the Prot1/Thaw-Lyse system.
• Assessment of early T-cell activation: This workflow involves a rapid, 15-min stimulation length using a proprietary T-cell activator that provides TCR engagement and co-stimulation.
• Expertise required: Intended for users experienced in surface and intracellular antibody staining techniques for cytometric applications.
Keywords: Helios CyTOF (Helios CyTOF)Graphical overview
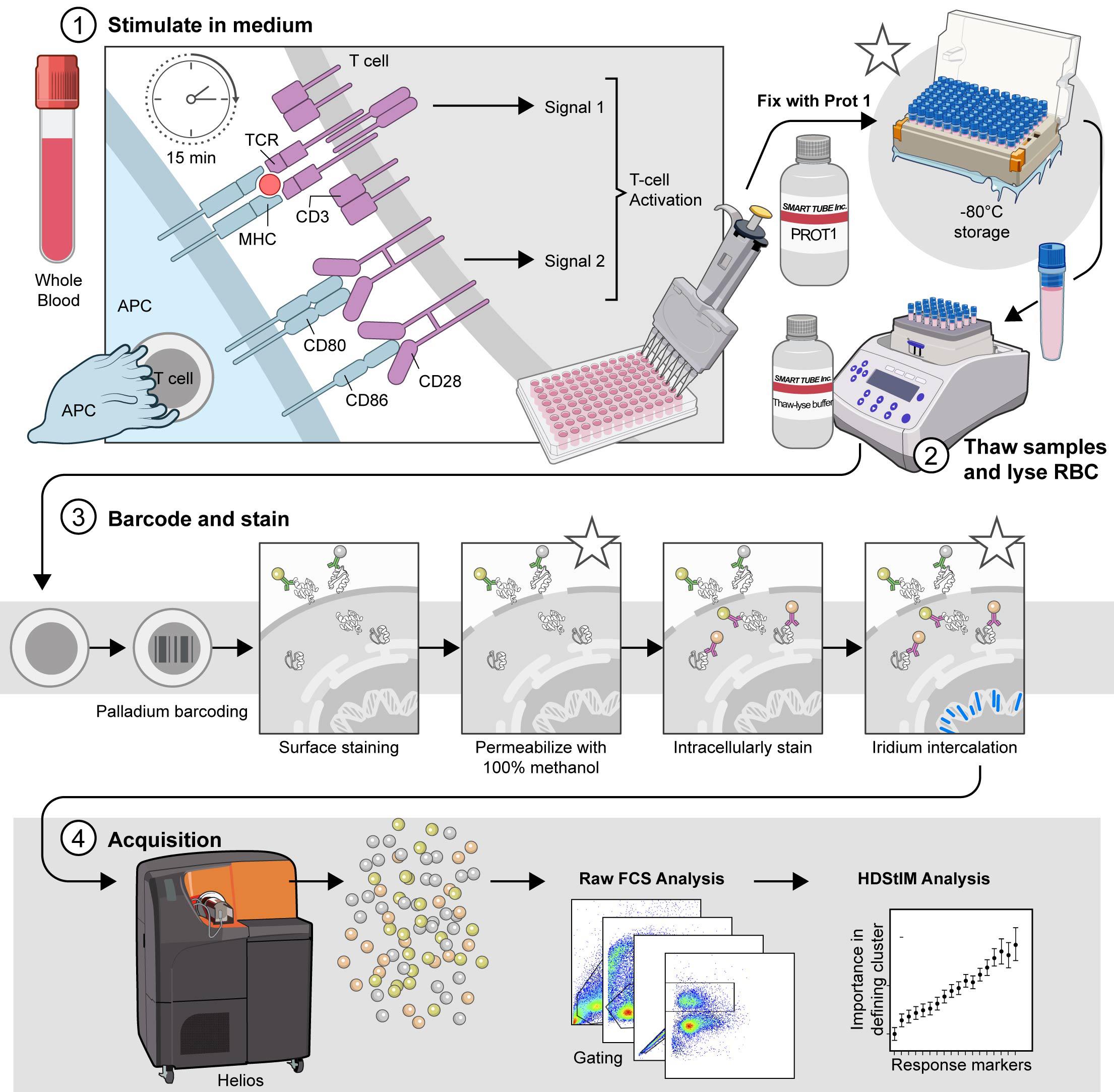
The comprehensive T-cell Phospho-CyTOF pipeline. This schematic illustrates the established pipeline for high-dimensional analysis of T-cell phosphorylation using mass cytometry, outlining the critical steps from sample preparation to data acquisition. 1) Stimulate in complete RPMI (cRPMI). Whole blood is stimulated to induce T-cell activation and subsequent phosphorylation events. After 15 min of stimulation, the cells are fixed with Prot1 to preserve cellular signaling states. (*) The fixed samples can be stored at -80 °C for later processing. Stimulated and fixed samples may be stored at -80 °C for approximately 6 months to one year prior to processing. 2) Thaw samples and lyse red blood cells (RBCs). When ready for downstream analysis, fixed samples are thawed and treated with Thaw-Lyse reagent to remove red blood cells. 3) Barcode and stain. Live cells are palladium-barcoded following the manufacturer's specifications to permit multiplexed analysis. Subsequently, cells undergo surface staining with metal-conjugated antibodies. Following surface staining, cells are permeabilized with 100% methanol on wet ice to allow access to intracellular antibodies. (*) The samples can be stored at -80 °C for 4–6 weeks in 100% methanol prior to intracellular staining. When ready, the samples are rehydrated and intracellularly stained with additional metal-conjugated antibodies targeting phosphorylated proteins and intracellular markers. (*) Finally, the cells are incubated overnight at 4 °C with an iridium intercalation solution for DNA staining, which facilitates live/dead cell discrimination and doublet exclusion during acquisition. Avoid storing samples in iridium intercalation solution for more than 12 h before acquisition. 4) Acquire. Before acquisition, samples are resuspended in cell acquisition solution plus (CAS+) containing a 1:10 dilution of EQ4 beads and adjusted to a concentration that yields approximately 350 events per second (±50 events per second). Data are acquired using a Helios mass cytometer and transferred for subsequent analysis. Asterisks (*) indicate potential stopping points within the protocol, where samples can be safely stored before proceeding to the next step.
Background
T-cell signaling depends on protein phosphorylation to activate and regulate signaling cascades that influence T-cell activation, differentiation, and effector function. This process begins immediately when antigens presented by antigen-presenting cells (APCs) bind to the T-cell receptor (TCR). This binding results in the phosphorylation of the receptor's intracellular domain, triggering a series of phosphorylation events along multiple pathways, including the MAPK/ERK kinase (MEK), protein kinase C (PKC), nuclear factor-κB (NF-κB), and JAK/STAT pathways. Despite advances in understanding T-cell signaling, there has been limited progress in characterizing the role of protein phosphorylation in T-cell fate and function at the single-cell level. This limitation is primarily due to technical challenges, including but not limited to the biological complexity of T cells, the need for advanced analysis techniques to handle high-dimensional data, and complications in experimental design (see Figure 1). TCR activation is crucial for the effectiveness of T-cell-targeted immunotherapies; however, current assays are insufficient for fully elucidating the phosphorylation status of key targets downstream of the TCR in patient samples. Furthermore, leveraging technologies that enable high-dimensional analysis of TCR signaling at the single-cell level could yield important insights into disorders associated with impaired T-cell function.
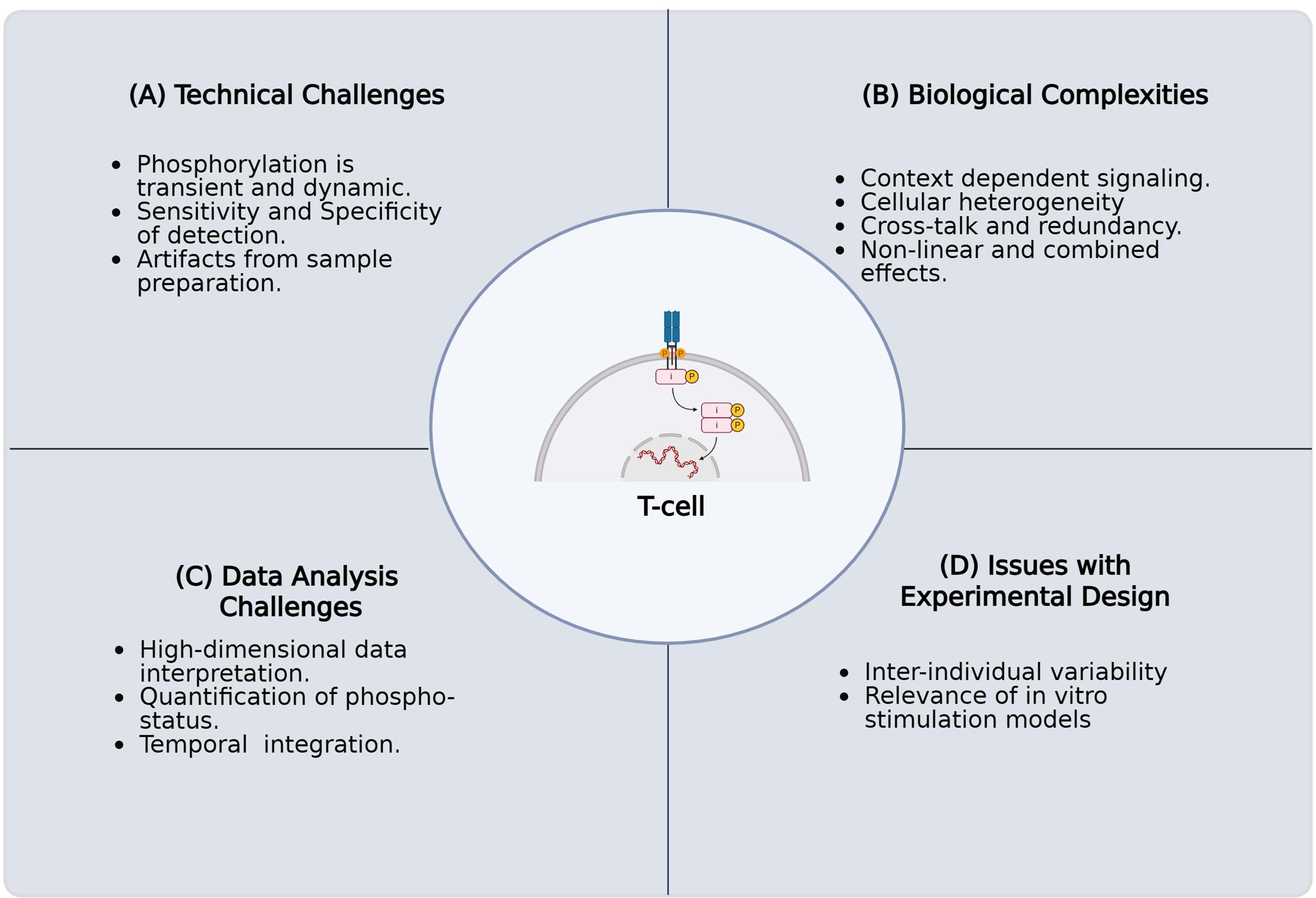
Figure 1. Challenges in characterizing protein phosphorylation and its impact on T-cell fate and function in primary human samples. The key challenges encountered when investigating the intricate role of protein phosphorylation in T-cell signaling pathways using primary human samples. These complications arise for several reasons. (A) Technical challenges involving the inherent biological complexity of T cells, including their diverse subsets, dynamic activation states, and rare antigen-specific populations. (B) Biological complexities, including context-dependent signaling, cellular heterogeneity, crosstalk, and nonlinear combined effects. (C) Challenges in high-dimensional data analysis, necessitated by single-cell resolution and multiomics approaches, present difficulties in data integration, normalization, noise reduction, and interpretation. (D) Issues with experimental design, such as execution, acquisition of sufficient numbers of quality primary human T cells, selecting appropriate controls, addressing donor variability, and confirming inter-experimental reproducibility. Overcoming these technical limitations is critical for a comprehensive understanding of T-cell biology and function.
TCR signaling is crucial for the activation, proliferation, and differentiation of naïve T cells into effector and memory subsets [3]. The activation of naïve T cells involves three key steps. First, an antigen is presented by the major histocompatibility complex (MHC) on antigen-presenting cells (APCs) and binds to the TCR, initiating a signaling cascade. This engagement also activates T-cell co-stimulatory receptors, which bind to their corresponding ligand receptors on APCs. For instance, the CD28 receptor on T cells interacts with the B7.1 or B7.2 ligands on APCs as a response to TCR activation. These interactions enhance T-cell activation and promote their proliferation. The third step is differentiation, which is driven by the secretion of autocrine and paracrine cytokines within the microenvironment. Together, these external signals influence intracellular processes that lead to changes in gene expression, ultimately shaping the T-cell response. Differential T-cell activation states can be investigated by analyzing intracellular protein phosphorylation following TCR stimulation. In patient samples with immunodeficiencies or those that have received T-cell therapies, understanding the depth of T-cell function can be achieved by examining phosphorylation patterns downstream of TCR signaling. However, little is known about how phosphorylation patterns vary in response to different T-cell activation states at the single-cell level. Therefore, developing a workflow to assess single-cell phosphorylation profiles of important signaling proteins after T-cell activation will be vital for defining the molecular mechanisms that drive T-cell function or dysfunction in patient samples.
Mass cytometry by time-of-flight (CyTOF) combines the principles of flow cytometry with mass spectrometry, making it a powerful, high-dimensional technique for single-cell protein analysis. This technology excels in assessing immunophenotypes and immune cell activation, making it integral to investigating immune status in complex human samples [4]. Unlike fluorescence-based flow cytometry, CyTOF employs heavy metal–tagged antibodies, typically from the lanthanide series, for protein detection. Upon acquisition, a sample is first nebulized and then introduced into an argon plasma, which vaporizes the cells and ionizes the metal tags into an ion cloud. Next, a time-of-flight mass spectrometer separates and measures the ions based on their mass-to-charge ratio. Each metal isotope is detected in a distinct channel, resulting in minimal or no overlap, remediating the issue of spectral overlap experienced with flow cytometric methods. This unique capability enables the simultaneous, high-resolution analysis of over 40 protein targets, including both intracellular and extracellular proteins, as well as those with post-translational modifications. CyTOF's increased capacity for target staining and minimal metal overlap enhances the likelihood of identifying rare or unexpected cell populations and capturing intracellular responses, even with limited sample material. Furthermore, this technology excels at characterizing immune cell activity within their native setting, revealing levels of heterogeneity, protein expression, and cellular complexity previously missed. Further, CyTOF enables the simultaneous investigation of differential signaling patterns across multiple pathways, such as those activated downstream of the TCR, which allows for the study of phosphorylation status. In comparison with alternative techniques, CyTOF excels in single-cell functional characterization of immune status through differential phosphorylation.
Fernandez and Maecker introduced the application of CyTOF to human blood samples in their Bio-Protocol publication, which describes a workflow for cytokine-stimulated phosphoflow of whole blood [1]. To achieve T-cell activation, the Stanford Human Immune Monitoring Core (HIMC) protocol, which utilizes an anti-CD3 antibody [5] and Protein A, was used as a benchmark for TCR activation. This method does not provide co-stimulation, which may lead to T-cell anergy and skewed results regarding T-cell activation. Currently, there is limited information in the literature regarding the in vitro stimulation of acute T-cell activation and the expected phosphorylation patterns in primary human T cells using endogenous stimuli that provide both required signals for activation. To enhance existing protocols, we utilized the framework established by Fernandez and Maecker [1] to develop a method for investigating T-cell activation and phosphorylation in response to more endogenous stimuli, specifically the combination of anti-CD3 antibody and anti-CD28 antibody, in whole blood. This approach may offer insights into the dynamics of T-cell function. Also, the use of "whole blood biopsies" as a less invasive method to study changes in the immune landscape longitudinally throughout disease progression and treatment can complement the spatial resolution provided by tissue analysis. In cases of immune dysregulation or cancer, high-dimensional techniques, such as a T-cell phospho-CyTOF pipeline, can assist in predicting viable treatment options and patient responses to therapeutic interventions. The ability to evaluate the functions of cytokine and antigen receptors, as well as alternative signaling pathways, in whole blood may yield valuable information about T-cell status with minimal invasiveness. Therefore, the innovation of technologies that enhance the throughput and resolution of these assays is essential.
Materials and reagents
Biological materials
1. Human whole blood collected by venipuncture and preserved in sodium heparin (green top) tubes (NIH Clinical Center Blood Bank)
2. Jurkat, clone E6-1 (ATCC, catalog number: TIB-152)
Reagents
1. IFNα (PBL, catalog number: 11105-1)
2. Lipopolysaccharide (LPS) from Salmonella enterica serotype enteritidis, γ-irradiated, BioXtra, suitable for cell culture (Sigma-Aldrich, catalog number: L7770-1MG)
3. Anti-human CD3 (clone UCHT1) (BD Biosciences, catalog number: 555329)
4. Protein-A (Sigma-Aldrich, catalog number: P6031-1MG)
5. ImmunoCult Human CD3/CD28 T-cell activator (StemCell Technologies, catalog number: 10971)
6. RPMI 1640 + GlutaMax (Thermo Fisher Scientific, catalog number: 61870036)
7. Heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific, catalog number: A5670801)
8. Dulbecco’s modified PBS without Ca2+/Mg2+ (Thermo Fisher Scientific, catalog number: 14190094)
9. Penicillin-Streptomycin (Pen/Strep) (10,000 U/mL) (Thermo Fisher Scientific, catalog number: 15140122)
10. Proteomic Stabilizer 1 (Prot1) (SmartTube Inc., catalog number: 501351691)
11. Thaw/lyse concentrate (SmartTube Inc., catalog number: 501351696)
12. Methanol (MeOH) ACS reagent, ≥99.8%, 1 L (Sigma-Aldrich, catalog number: 179337-1L)
13. Water, molecular biology grade (Quality Biological, catalog number: 351-029-101)
14. Maxpar® 10× Barcode perm concentrate (50 mL) (Standard BioTools, catalog number: 201057)
15. Maxpar® PBS (500 mL) (Standard BioTools, catalog number: 201058)
16. Maxpar® cell staining buffer (CSB) (500 mL) (Standard BioTools, catalog number: 201068)
17. Cell-IDTM 20-Plex Pd Barcoding kit (Standard BioTools, catalog number: 201060)
18. Cell-IDTM Intercalator-Ir, 12.5 μM (500 μL) (Standard BioTools, catalog number: 201192C)
19. EQ Four Element calibration beads (EQ4 beads) (100 mL) (Standard BioTools, catalog number: 201078)
20. Maxpar® fix and perm buffer (100 mL) (Standard BioTools, catalog number: 201067)
21. Maxpar® cell acquisition solution plus (CAS+) for CyTOF® XT (1,000 mL) (Standard BioTools, catalog number: 201244)
22. Maxpar® water (500 mL) (Standard BioTools, catalog number: 201069)
23. CyTOF tuning solution (250 mL) (Standard BioTools, catalog number: 201072)
Solutions
1. Stimulation media (cRPMI) (see Recipes)
2. 1× Thaw/lyse buffer (see Recipes)
3. 1× Barcoding (BC) buffer (see Recipes)
4. Iridium intercalation solution (see Recipes)
5. 1:10 EQ4 beads (see Recipes)
Recipes
1. Stimulation media (cRPMI)
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 + GlutaMax | 89% | 445 mL |
| Heat-inactivated FBS | 10% | 50 mL |
| Pen/strep | 1% | 5 mL |
| Total | 500 mL |
Filter-sterilize the stimulation media through a 500 mL 0.22 μm filtration unit. Stimulation media can be prepared up to one month before use and stored at 4 °C.
2. 1× Thaw/lyse buffer
| Reagent | Final concentration | Volume |
|---|---|---|
| Molecular biology–grade water | 49.95 mL | |
| Thaw/lyse concentrate (1,000×) | 1× | 0.05 mL |
| Total | 50.00 mL |
Filter-sterilize the 1× thaw/lyse buffer through a 500 mL 0.22 μm filtration unit. 1× thaw/lyse buffer can be prepared and stored at room temperature (RT) for up to one month from the preparation date.
3. 1× Barcoding (BC) buffer
| Reagent | Final concentration | Volume |
|---|---|---|
| MaxPar PBS | 45mL | |
| Maxpar® 10× barcode perm concentrate | 1× | 5 mL |
| Total | 50 mL |
1× barcoding buffer can be stored at 4 °C and used for up to one month from the preparation date.
4. Iridium intercalation solution
| Reagent | Final concentration | Volume |
|---|---|---|
| Maxpar® fix and perm buffer | 3.998 mL | |
| Cell-IDTM Intercalator-Ir, 12.5 μM | 2,000× | 0.002 mL |
| Total | 4.0 mL |
Safe handling of iridium requires strict adherence to lab safety protocols for heavy metals. Specific iridium compounds are toxic, corrosive, or irritating. Wear appropriate personal protective equipment and work in a fume hood when applicable.
Cell-IDTM intercalator-Ir is light-sensitive. Ensure that any aliquots or solutions are protected from light.
Cell-IDTM intercalator-Ir should be aliquoted and frozen at -20 °C immediately after thawing. After a second thaw, aliquots of Cell-IDTM intercalator-Ir should be discarded.
The iridium intercalation solution should be prepared immediately before use and stored on wet ice.
5. 1:10 EQ4 beads
| Reagent | Final concentration | Volume |
|---|---|---|
| Maxpar® Cell acquisition solution plus (CAS+) for CyTOF XT | 9 mL | |
| EQ Four Element calibration beads | 10× | 1 mL |
| Total | 10 mL |
The EQ Four Element calibration beads require vigorous shaking by hand for at least 30 s prior to use. Do not vortex the EQ4 beads, as vortexing fails to resuspend the beads into solution. The 1:10 EQ4 beads in CAS+ should be prepared immediately before use and stored on ice.
Laboratory supplies
1. 25 mL sterile reservoirs (Thermo Fisher Scientific, catalog number: 95128095)
2. DNA LoBind tube 2.0 mL (Eppendorf, catalog number: 022431048)
3. Safe-Lock tubes 1.5 mL, natural (Eppendorf, catalog number: 022363212)
4. Matrix 0.5 mL 2D screw tubes PP, V-bottom, w/ clear caps (Thermo Fisher Scientific, catalog number: 3744)
5. Matrix 0.5 mL 2D screw tubes PP, V-bottom, w/ yellow caps (Thermo Fisher Scientific, catalog number: 3744YEL)
6. Matrix 0.5 mL 2D screw tubes PP, V-bottom, w/ blue caps (Thermo Fisher Scientific, catalog number: 3744BLU)
7. Matrix 0.5 mL 2D screw tubes PP, V-bottom, w/ red caps (Thermo Fisher Scientific, catalog number: 3744RED)
8. Matrix 0.5 mL 2D screw tubes PP, V-bottom, w/ pink caps (Thermo Fisher Scientific, catalog number: 3744PIN)
9. 14 mL polystyrene round-bottom tube (Corning, catalog number: 352051)
10. 5 mL polystyrene round-bottom tube 12 × 75 mm style (Falcon, catalog number: 352058)
11. 5 mL polystyrene round-bottom tube with cell strainer cap (Falcon, catalog number: 352235)
12. 50 mL polypropylene conical tube 30 × 115 mm style (Corning, catalog number: 352070)
13. LTS 1,200 μL filter 768/4 tips (Rainin, catalog number: 17007084)
14. TR LTS 1,000 μL filter 768/4 tips (Rainin, catalog number: 17014967)
15. Pipette tips RT LTS 300 μL 768A/8 (Rainin, catalog number: 30389253)
16. RTS LTS 200 μL F 960A/10 (Rainin, catalog number: 30389239)
17. Dualfilter 200 μL, PCR clean/sterile (Eppendorf, catalog number: 022491296)
18. P20 LTS pipette tips (Rainin, catalog number: 17014392)
19. Sterile 5 mL serological pipettes (VWR Scientific, catalog number: 170355N)
20. Sterile 10 mL serological pipettes (VWR Scientific, catalog number: 89130-898)
21. Nunc plate seals (ThermoFisher Scientific, catalog number: AB0718)
22. 96-well deep well 2 mL plate (Fisher Brand, catalog number: 12-566-121)
23. 96-well V-bottom plate with lid polystyrene TC-treated (Corning, catalog number: 3894)
24. 96-well U-bottom plate with lid polystyrene TC-treated (Corning, catalog number: 3799)
25. C-Chip (inCyto, catalog number: DHC-N01-5)
Equipment
1. Helios CyTOF (Standard BioTools, catalog number: PN 400250 A7)
2. Thermo Sorvall Legend XTR Refrigerated Centrifuge (Marshall Scientific, catalog number: TSO-LEGXTR)
3. CorningTM Mini Microcentrifuge, 100–240 V (Fisher Scientific, catalog number: 07-203-954)
4. -86 °C upright freezer (Phcbi, catalog number: MDFDU702VHPA)
5. -20 °C K2 Scientific Laboratory Undercounter Freezer (Fisher Scientific, catalog number: K204SDF)
6. 4 °C TSX Series High-Performance Lab Refrigerator (ThermoFisher Scientific, catalog number: TSX3005SD)
7. EppendorfTM Centrifuge 5430 R, Refrigerated Microcentrifuge (Fisher Scientific, catalog number: 13-690-005)
8. Water bath (Corning, catalog number: 6783)
9. Pipet-Lite Pipette, Universal SL-1000XLS+ (Mettler Toledo, catalog number: 17014407)
10. Pipet-Lite Pipette, Universal SL-200XLS+ (Mettler Toledo, catalog number: 17014391)
11. Pipet-Lite LTS Pipette L-20XLS+ (Mettler Toledo, catalog number: 17014392)
12. Pipet-Lite LTS Pipette L-10XLS+ (Mettler Toledo, catalog number: 17014388)
13. Pipet-Lite LTS Pipette L-2XLS+ (Mettler Toledo, catalog number: 17014393)
14. Pipet-Lite Pipette Multi L12-1200XLS+ (Mettler Toledo, catalog number: 17014497)
15. Pipet-Lite Multi Pipette L12-300XLS+ (Mettler Toledo, catalog number: 17013811)
16. Pipet-Lite Multi Pipette L12-200XLS+ (Mettler Toledo, catalog number: 17013810)
17. CorningTM 8-Channel Adapter for Vacuum Aspirator (Corning, catalog number: 4931)
18. Pipet-Aid® XP (Drummond Scientific Company, catalog number: 4-000-101)
19. Eppendorf® Thermomixer® FP (Millipore Sigma, catalog number: EP5385000024)
20. Vortex Genie-2 (Millipore Sigma, catalog number: Z258423)
21. FormaTM Series II Water-Jacketed CO2 Incubator (Thermo Fisher, catalog number: 3131)
22. 1300 Series Class II, Type A2 Biological Safety Cabinet Packages (Thermo Fisher, catalog number: 1323TS)
23. Microscope (Olympus, catalog number: CKX53)
Software and datasets
1. CyTOF Software (Standard BioTools, version 7.1), https://www.fluidigm.com/software
2. Excel (Microsoft, version 2408), license required, https://www.microsoft.com/en-us/microsoft-365/excel
3. FlowJo (TreeStar, version 10.10), license required, https://www.flowjo.com/solutions/flowjo/downloads/
4. GraphPad Prism (GraphPad Software LLC., version 9.5.0), license required, https://www.graphpad.com/scientific-software/prism/
5. RStudio (Posit Software, PBC, version 2024.09.1+394), no license required, https://posit.co/download/rstudio-desktop/
6. HDStIM (GitHub) [6], no license required, https://doi.org/10.1101/2022.11.14.516371
Procedure
文章信息
稿件历史记录
提交日期: Jul 13, 2025
接收日期: Sep 7, 2025
在线发布日期: Sep 18, 2025
出版日期: Oct 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Ramos, I. E., Hawley, T. S., Rose, K., Matthiasardottir, B., Farmer, R., Han, K. L., Toborek, M., Douagi, I., Jones, G. N. and Cherry, J. M. (2025). High-Dimensional Phospho-CyTOF Characterization of T-Cell Activation Responses in Whole Blood. Bio-protocol 15(20): e5473. DOI: 10.21769/BioProtoc.5473.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
系统生物学 > 蛋白质组学 > 磷酸化蛋白质组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link








