- EN - English
- CN - 中文
Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth
耐盐耐旱细菌分离株中生长素、铁载体和氰化氢产量的定量评估及其对黄瓜生长的作用
发布: 2025年10月05日第15卷第19期 DOI: 10.21769/BioProtoc.5471 浏览次数: 1356
评审: Jose Roberto TorresAnonymous reviewer(s)

相关实验方案

基于 Emu 的可重复计算流程:在高性能计算集群上实现高保真土壤—植物微生物组解析
Henrique M. Dias [...] Christopher Graham
2026年01月20日 441 阅读
Abstract
Salt-tolerant bacteria can enhance plant growth through various mechanisms, including the production of auxin, siderophores, hydrogen cyanide, and the solubilization of insoluble phosphate. This study investigated the production of these growth-stimulating factors by salt- and drought-tolerant bacteria isolated from the arid and saline farmlands of Jiroft. Initially, we screened for bacterial strains that exhibited the highest levels of these factors. We then evaluated their effects on improving the growth indices of cucumber seedlings. Additionally, we optimized the protocols for isolating auxin, siderophores, hydrogen cyanide, and phosphate solubilization, which can also be applied to other host rhizobacteria to assess their growth-promoting compounds.
Key features
• The most resistant bacterial isolates to salinity and drought are identified by adding salt and polyethylene glycol to the culture medium in laboratory conditions.
• This protocol can be used to evaluate the production levels of IAA, siderophore, hydrogen cyanide, and phosphate solubilization by salt- and drought-tolerant bacteria.
• This protocol can also be used to evaluating the plant growth–promoting ability of salt- and drought-tolerant bacteria under greenhouse conditions.
• This protocol can be applied to other host rhizobacteria to assess their growth-promoting compounds.
Keywords: Auxin (生长素)Graphical overview
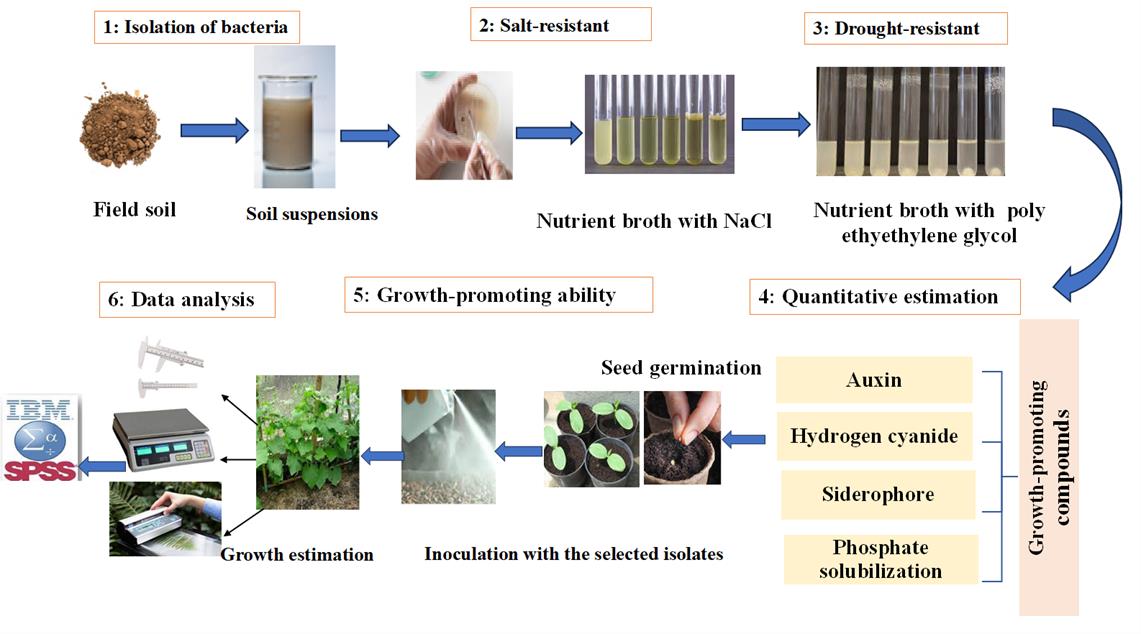
Graphical representation of the protocol for quantitative estimation of growth-promoting compounds in halotolerant bacterial isolates for cucumber growth. 1) Isolation of bacteria. 2) Screening for salt-resistant bacteria. 3) Screening for drought-resistant bacteria. 4) Quantitative estimation of auxin, siderophore, and hydrogen cyanide production, as well as phosphate solubilization activity. 5) Assessing the effect of superior isolates on cucumber seedling growth. 6) Data analysis. Arrows indicate the sequence of the experimental procedure.
Background
In recent years, the use of plant growth–promoting rhizobacteria (PGPR) has emerged as a form of biofertilizer [1]. These bacteria enhance plant resistance to environmental stresses and improve growth and yield through various mechanisms, including nitrogen fixation, solubilization of insoluble phosphate, production of siderophores, synthesis of plant hormones like auxin, and reduction of ethylene concentration [2]. Indole-3-acetic acid (IAA) is one of the most active plant hormones. It plays a crucial role in cell division and elongation, enhances the surface area and weight of root hairs, and promotes the development of lateral roots [3]. In young stems, IAA helps in the apical dominance and stimulates growth [4]. Moreover, IAA-producing PGPRs are reported to boost nutrient uptake and chlorophyll content [5]. Siderophores produced by plant growth–promoting rhizobacteria (PGPR) in response to iron deficiency enhance iron uptake by solubilizing and chelating iron. They form ferric–siderophore complexes that increase iron availability, even at very low concentrations, thereby supporting iron supply and promoting plant growth [6]. According to certain study findings, different bacteria, like Pseudomonas and Bacillus, can produce siderophores [7–10]. Using the CAS (Chrome-Azurol S) method for initial selection of potential iron-solubilizing isolates is an effective screening method [11]. Moreover, hydrogen cyanide (HCN), produced by many rhizobacteria [12], may affect plant establishment or inhibit the development of plant disease [13]. Some studies suggest that HCN participates in geochemical processes within the substrate, such as metal chelation, which indirectly increases nutrient availability for both rhizobacteria and their plant hosts [14]. Phosphate-solubilizing activity has been reported in many PGPR genera [15]. It is estimated that about 95%–99% of phosphorus in soil is unavailable to plants [16], so the use of such bacteria can replace phosphate fertilizers. Reports indicate that Bacillus species enhance plant growth significantly by producing phytohormones and siderophores, as well as by solubilizing phosphate. This results in increased stem length and total dry weight [17]. Bacteria isolated from extremely saline and hot soils by Gil et al. [18] have been shown to enhance root size under osmotic stress in Medicago sp. Given the widespread presence of saline soils in Iran and the significant agricultural importance of Jiroft County, this study aims to evaluate the production levels of IAA, siderophore, hydrogen cyanide, and phosphate solubilization by salt- and drought-tolerant bacteria. Additionally, it will assess the impact of these bacteria on the growth of cucumber seedlings. This research marks the first investigation in Jiroft County—the agricultural hub of Iran—focused on promoting the growth of the region’s key agricultural product. If the results are positive, these findings could potentially be applied as a biological fertilizer at the farm level.
Materials and reagents
Biological materials
1. Halotolerant PGPR bacterial strains
Note: Our strains were isolated from field soil samples at a depth of 30 cm in the arid and saline regions of Jiroft (Kanarsandal, Jazsaleh, Anbarabad, and Karimabad areas) in Kerman province, Iran. All isolates were found to belong to the Bacillus genus, with isolate C8 identified as the most effective. Following PCR amplification of the 16S rDNA, an approximate 1,500 bp band was observed on an agarose gel. Amplicon sequencing was conducted by Bioneer Corporation in South Korea. After sequencing the amplified products, BLAST alignment was performed on NCBI to find a strain with a high degree of homology. The molecular weight of these sequence fragments matched that of the bacterial 16S rDNA fragment. Based on the BLAST results and the phylogenetic tree analysis, the C8 isolate was identified as Bacillus subtilis, as its amplicon sequence shared 99%–100% similarity with Bacillus subtilis.
Bacterial codes: K: Kanarsandal; C: Karimabad; J: Jazsaleh; and A: Anbarabad.
2. Cucumber seeds (Royal cultivar) were obtained from the Agricultural and Natural Resources Research Center of Southern Kerman (Iran)
Reagents
1. Glycine (Sigma-Aldrich, catalog number: 56-40-6)
2. L-tryptophan (Sigma, catalog number: 65072-00-6)
3. Casamino acids (Sigma, catalog number: 65072-00-6)
4. K2HPO4 (Sigma, catalog number: 7758-11-4)
5. MgSO4·7H2O (Sigma, catalog number: 10034-99-8)
6. Glycerin (Sigma, catalog number: 0770525)
8. Tryptone (OXOID, catalog number: LP0042)
9. Agar (Sigma-Aldrich, catalog number: 9002-18-0)
10. Potassium chloride (KCl) (Merck, catalog number: 1.93238.0521)
11. Sodium chloride (NaCl) (Merck, catalog number: 1.93606.0521)
12. Ammonium sulfate [(NH4)2SO4] (Sigma, catalog number: 7783-20-2)
13. Yeast extract (OXOID, catalog number: LP0021)
14. Tricalcium phosphate [Ca3(PO4)2] (Sigma, catalog number: 7758-87-4)
15. Glucose (Sigma, catalog number: 492-62-6)
16. Iron sulfate (FeSO4·7H2O) (Sigma-Aldrich, catalog number: 7782-63-0)
17. Manganese sulfate (MnSO4·H2O) (Sigma-Aldrich, catalog number: 10034-96-5)
18. FeCl3 (Sigma, catalog number: 7705-08-0)
19. Chrome azurol S (CAS) (Sigma, catalog number: 1667-99-8)
20. HTDMA (Sigma, catalog number: 10424-65-4)
21. Piperazine solution (Merck, catalog number: 807325)
22. Perchloric acid (HClO4) (Sigma, catalog number: 1005190510)
23. Soytone (Sigma, catalog number: 91079-46-8)
24. 2,2′ dipyridyl (DIP) (Sigma, catalog number: 366-18-7)
25. Beef extract (Sigma, catalog number: 68990-90-0)
26. Peptone (Sigma, catalog number: 100209-45-8)
27. Sodium hypochlorite (Nice, catalog number: S2195)
28. Ethanol (Biopack, catalog number: 2000165400)
29. KOH (Sigma, catalog number: 497-19-8)
30. Sterile distilled water
31: Sodium carbonate (Sigma, catalog number: 65072-00-6)
32. Picric acid (Sigma, catalog number: 501840460)
33. Polyethylene glycol (Sigma, catalog number: 25322-68-3)
34. KH2PO4 (Merck, catalog number: 1.93205.0521)
35. Minimal agar medium (Sigma, catalog number: 45-79332-500G-F)
Solutions
1. Nutrient broth medium (see Recipes)
2. Nutrient agar (NA) (see Recipes)
3. Iron (III) solution (see Recipes)
4. MKB medium (see Recipes)
5. Minimal agar medium (see Recipes)
6. Minimum iron medium (see Recipes)
7. Tryptic soy agar (TSA) medium (see Recipes)
8. Picosky solid culture medium (see Recipes)
9. CAS solution (see Recipes)
10. Salkowski reagent (see Recipes)
11. Ethanol 70% (see Recipes)
Recipes
1. Nutrient broth (NB) (1 L)
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| Beef extract | 1.0% (w/v) | 1.0 g |
| Peptone | 1% (w/v) | 1 g |
| Yeast extract | 2.0% (w/v) | 2.0 g |
| NaCl | 0.5% (w/v) | 0.5 g |
| Sterile distilled water | n/a | 1 L |
Final pH 7.
2. Nutrient agar (NA) (1 L)
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| Nutrient broth | 8% (w/v) | 8 g |
| Agar | 15% (w/v) | 15 g |
| Sterile distilled water | n/a | 1 L |
3. Iron (III) solution
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| FeCl3·6H2O | 1 mmol/L | 1 mmol |
| HCl | 10 mmol/L | 10 mmol |
| Sterile distilled water | - | 1 L |
4. MKB medium (1 L)
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| K2HPO4 | 2.5 g/L | 2.5 g |
| MgSO4·7H2O | 2.5 g/L | 2.5 g |
| Glycerin | 15 mL/L | 15 mL/L |
| Casamino acids | 5.0 g/L | 5.0 g |
| Sterile distilled water | - | 1 L |
Final pH 7.2.
5. Minimal agar medium (1 L)
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| Dextrose | 1% (w/v) | 1 g |
| Dipotassium phosphate | 7% (w/v) | 7 g |
| Monopotassium phosphate | 2% (w/v) | 2 g |
| Sodium citrate | 0.5% (w/v) | 0.5 g |
| Magnesium sulphate | 0.1% (w/v) | 0.1 g |
| Ammonium sulphate | 1% (w/v) | 1 g |
| Agar | 15% (w/v) | 15 g |
| Sterile distilled water | - | 1 L |
Final pH 7.
6. Minimum iron medium (1 L)
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| Tryptone | 0.1% | 0.1 mL |
| Casamino acids | 0.1% | 0.1 mL |
| Tryptophan (CAS+Trp( | 0.1 mg/mL | 0.1 mg |
| Yeast extract | 0.1% | 0.1 mL |
| DIP | 0.1% | 0.1 mL |
| Sterile distilled water | - | 1 L |
Final pH 6.85.
7. Tryptic soy agar (TSA) medium (1 L)
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| Tryptone | 15% (w/v) | 15 g |
| Soytone | 5% (w/v) | 5 g |
| NaCl | 5% (w/v) | 5 g |
| Agar | 5% (w/v) | 5 g |
| Sterile distilled water | n/a | 1 L |
8. Picosky solid culture medium (1 L)
| Reagents | Final concentration | Quantity or Volume |
|---|---|---|
| Glucose | 10% (w/v) | 10 g |
| Tricalcium phosphate | 5% (w/v) | 5 g |
| Yeast extract | 0.5% (w/v) | 0.5 g |
| Ammonium sulfate | 0.5% (w/v) | 0.5 g |
| Potassium chloride | 0.2% (w/v) | 0.2 g |
| Sodium chloride | 0.2% (w/v) | 0.2 g |
| Magnesium sulfate | 0.1% (w/v) | 0.1 g |
| Iron sulfate | 0.0003% (w/v) | 0.0003 g |
| Manganese sulfate | 0.0003% (w/v) | 0.0003 g |
| Agar | 10% (w/v) | 10 g |
| Sterile distilled water | n/a | 1 L |
Final pH 7.
9. CAS solution
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| 1 mM FeCl3 | 1.5% | 1.5 mL |
| 1 mM CAS | 7.5% | 7.5 mL |
| 4 mmol/L HTDMA | 50% | 50 mL |
| 1 M piperazine solution | 30% | 30 mL |
| Sterile distilled water | n/a | 100 mL |
10. Salkowski reagent
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| 35% perchloric acid (HClO4) | 49% | 49 mL |
| 0.5 M FeCl3 | 1% | 1 mL |
11. Ethanol 70%
| Reagents | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol 96% | 73% | 73 mL |
| Sterile distilled water | 27% | 27 mL |
Laboratory supplies
1. Sterile glass rod
2. Petri dishes, 90 mm (Abdos, catalog number: 3165077)
3. Microtips 200–1,000 μL (Tarson, catalog number: 521020)
4. Microtips 2–200 μL (Tarson, catalog number: 521010)
5. Microtips 0.2–10 μL (Tarson, catalog number: 521000)
6. Culture bottles (Tarsons, catalog number: 20080)
7. Fungicide captain (Wp50%)
8. Clean pots (21 × 16 × 16 cm)
9. Micropipettes (Eppendorf Research Plus, catalog number: 35016146026)
10. 2 mL microcentrifuge tubes (Eppendorf, catalog number: P10203)
11. 50 mL centrifuge tubes (Falcon, catalog number: 546024)
12. Filter papers (Whatman No. 1) (Sigma, catalog number: FP/310/AC)
13. Cotton (HiMedia, catalog number: LA1018)
14. Parafilm (Amcor, catalog number: PM-996)
15. Perlite (Keltech Energies Limited, catalog number: 27030090)
16. Household aluminum foil for multiple uses
17. 5 mL microcentrifuge tubes (Eppendorf, catalog number: 4092.4N)
Equipment
1. Spectrophotometer (Jenway, model: 6505)
2. Rotary shaker incubator (Biological Oxygen Demand, BOD)
3. Digital camera (Canon, model: DS126431)
4. mm-precision graduated ruler
5. Digital scale with accuracy of 0.001 g
6. Oven (DIAL 2.5 AMPS 240 V 600 W, catalog number: BARN3606-1CE)
7. Leaf scanner (Hewlett-Packard Scan Jet 7400C scanner)
8. Vortexer (VWR® Fixed Speed Vortex Mixer, catalog number: 10153-834)
9. Centrifuge (Eppendorf 5424R Microcentrifuge, catalog number: 05-401-205)
Software and datasets
1. ImageJ software (NIH, https://imagej.net/)
2. Excel (Microsoft Office 2016)
3. Photoshop graphical software (2025) (v. 26.10.0.7)
4. SPSS Statistics Software Version 26
Procedure
文章信息
稿件历史记录
提交日期: Jun 11, 2025
接收日期: Sep 1, 2025
在线发布日期: Sep 30, 2025
出版日期: Oct 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Fotoohiyan, Z. and Sardoei, A. S. (2025). Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth. Bio-protocol 15(19): e5471. DOI: 10.21769/BioProtoc.5471.
分类
植物科学 > 植物免疫 > 宿主-细菌相互作用
微生物学 > 微生物生理学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










