- EN - English
- CN - 中文
Combining Microwave Ablation With CAR-T-Cell Therapy in Tumor-Bearing Mouse Models
在荷瘤小鼠模型中联合应用微波消融与CAR-T细胞治疗
(*contributed equally to this work) 发布: 2025年10月20日第15卷第20期 DOI: 10.21769/BioProtoc.5470 浏览次数: 2333
评审: Pilar Villacampa AlcubierreAnonymous reviewer(s)
Abstract
Microwave ablation (MWA) is a thermal ablation technique widely used for local tumor control that has the added potential to stimulate systemic anti-tumor immunity. Although MWA alone rarely eliminates recurrent or metastatic disease, its ability to remodel the tumor microenvironment makes it a promising partner for adoptive cell therapies such as chimeric antigen receptor (CAR)-T cells. However, reproducible protocols for combining these approaches remain limited. This protocol describes the integration of MWA with CAR-T therapy in tumor-bearing mouse models. Human hepatocellular carcinoma cell lines (Hep3B and SK-HEP-1) are inoculated subcutaneously into NOG mice to establish tumors. Localized MWA is performed at adjustable power and duration to induce partial or complete ablation. At defined intervals following MWA, CAR-T cells derived from healthy donor T cells and transduced with a lentiviral vector are injected intravenously. This experimental design uniquely separates MWA and CAR-T delivery, enabling precise evaluation of thermal preconditioning effects on the tumor microenvironment and subsequent CAR-T activity. By combining localized ablation with adoptive immunotherapy, the protocol provides a translationally relevant platform to optimize treatment timing, enhance CAR-T efficacy in solid tumors, and address key barriers in tumor immunology and cancer therapy.
Key features
• Practical protocol to investigate MWA and CAR-T-cell combination therapy in mouse tumor models.
• Includes detailed procedures for tumor cell preparation, subcutaneous inoculation, MWA treatment, and CAR-T-cell administration.
• Novel protocol design separates MWA and CAR-T delivery, allowing rigorous analysis of preconditioning effects on the tumor microenvironment and therapy response.
• Offers translational relevance for improving CAR-T therapy in solid tumors, addressing a critical barrier to clinical application.
Keywords: Microwave ablation (微波消融)Graphical overview
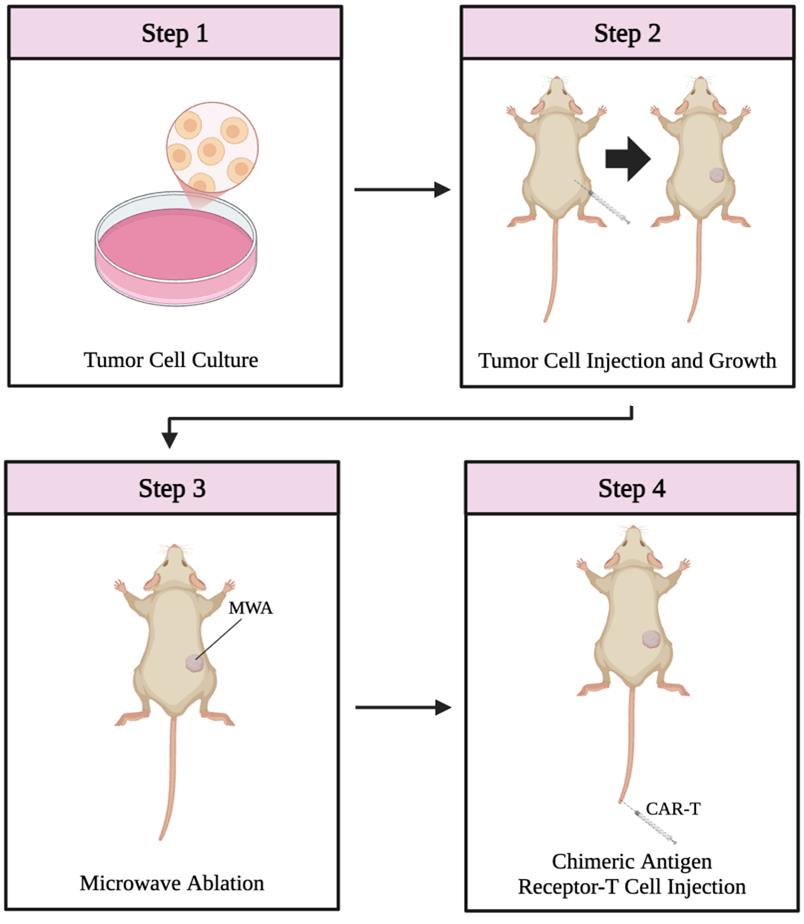
Overview of the combination therapy protocol. The protocol consists of four main steps: tumor cell culture (step 1, day 0), subcutaneous tumor establishment in mice (step 2, day 8), microwave ablation (step 3, days 15–24), and CAR-T-cell administration (step 4).
Background
Microwave ablation (MWA) is a thermal ablation technique for the treatment of tumors. In this procedure, a needle delivers microwave radiation to a tumor site, causing localized heating [1,2]. This process induces necrosis of surrounding tissues, resulting in cell damage at the membrane and subcellular levels and leading to decreased tumor burden [1]. Studies using animal models have demonstrated that MWA holds promise for treating various solid tumors, including liver, lung, bone, and renal masses [3]. In addition to its effectiveness, MWA is considerably less invasive than surgical approaches, typically requiring only a needle puncture rather than a full incision in most patients [4]. This minimally invasive nature results in shorter hospital stays, faster recovery times, and lower infection-related mortality rates [5]. As a result, MWA is increasingly being adopted as a viable clinical treatment for local tumor control [6,7].
Beyond its local cytotoxic effect, MWA has been shown to elicit an immune-mediated systemic response known as the abscopal effect. This phenomenon describes the regression of metastatic tumors at sites distant from the ablation, driven by immune activation resulting from post-MWA release of cytokines, chemokines, and neoantigens [8–13]. However, the abscopal effect alone is generally insufficient to eliminate secondary tumor sites completely [1,14]. Additionally, the high rate of tumor recurrence following MWA suggests that it may not be adequate as a standalone therapy for metastatic disease. Recent studies have shown that the immunostimulatory effects of MWA can complement immunotherapies such as chimeric antigen receptor (CAR)-T-cell therapy [15,16]. In particular, xenograft models of non-small cell lung cancer have demonstrated enhanced tumor regression when MWA is combined with CAR-T treatment [15,16]. This combination strategy has the potential to address both local and distant tumor sites more effectively than either modality alone.
Animal models, particularly mouse models, provide a valuable platform for investigating the synergistic potential of MWA and CAR-T-cell therapy before translation into clinical settings. However, protocols detailing the implementation of this combined approach remain limited. In this study, we present a methodology encompassing tumor cell culture, tumor inoculation, MWA administration, CAR-T cell infusion, and post-treatment monitoring in murine models, with the goal of establishing a standardized protocol for this promising therapeutic strategy.
Materials and reagents
Biological materials
1. Hep3B cells (ATCC, catalog number: HB-8064)
2. SK-HEP-1 cells (ATCC, catalog number: HTB-52)
3. CAR-T cells (generated in-house [16])
4. NOG (NOD/Shi-scid IL2rγnull) mice (Vital River, catalog number: 408)
Reagents
1. Trypsin (Corning, catalog number: MT25053CI)
2. Trypan blue (Corning, catalog number: MT25900CI)
3. Phosphate-buffered saline (PBS) (Invitrogen, catalog number: AM9624)
4. 0.25% trypsin-EDTA (Gibco, catalog number: 25200072)
5. Phenobarbital anesthesia (Covetrus, catalog number: 42494041503)
6. DMEM (Gibco, catalog number: 11885084)
7. RPMI-1640 (Gibco, catalog number: 61870036)
8. Fetal bovine serum (FBS) (Gibco, catalog number: A5670701)
9. Human recombinant IL-2 (PeproTech, catalog number: 200-02-50UG)
10. Penicillin-streptomycin (Gibco, catalog number:15140122)
11. Povidone iodine (Sigma-Aldrich, catalog number: 25655-41-8)
12. Ethanol (HYNAUT, catalog number: ZD119)
13. Carprofen (Yeasen, catalog number: 53634ES70)
14. Ophthalmic ointment (Fisher, catalog number: 50-218-8442)
15. T-Cell Isolation kit (StemCell Technologies, catalog number: 17951)
16. ImmunoCultTM human CD3/CD28 T cell activator (StemCell Technologies, catalog number: 17951)
Solutions
1. Tumor cell culture medium (see Recipes)
2. CAR-T-cell culture medium (see Recipes)
Recipes
1. Tumor cell culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM | n/a | 445 mL |
| FBS | 10% | 50 mL |
| Penicillin-streptomycin | 1% | 5 mL |
| Total | n/a | 500 mL |
Culture tumor cell lines, including Hep3B and SK-HEP-1, in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. Store prepared medium at 4 °C.
2. CAR-T-cell culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI-1640 | n/a | 445 mL |
| FBS | 10% | 50 mL |
| Recombinant IL-2 | 10 ng/mL | n/a |
| Penicillin-streptomycin | 1% | 5 mL |
| Total | n/a | 500 mL |
Culture CAR-T cells in RPMI-1640 supplemented with FBS, IL-2, and penicillin-streptomycin. Store prepared medium at 4 °C.
Laboratory supplies
1. Cell culture dish (Greiner Bio-One, catalog number: 628160)
2. Injection syringe with needle (23–25 G) (KDL, catalog number: 60017031)
3. Scalpel (Fisherbrand, catalog number: 08-925)
4. Tissue glue (Vetbond, catalog number: NC0735004)
5. Caliper (Fisherbrand, catalog number: 06-664-16)
6. Tissue adhesive (3M Vetbond, catalog number: 6606-65-1)
7. Hemocytometer (Bright-LineTM, catalog number: Z359629)
8. 15 mL conical tubes (Corning, catalog number: CLS430052)
9. Cryogenic vials (Corning, catalog number: CLS430659-500EA)
10. Cotton swabs (McKesson, catalog number: 13262-2S)
11. Heating pad (Braintree Scientific, catalog number: 50-195-4000)
12. Forceps (Fisherbrand, catalog number: 08-875)
13. Scissors (Fisherbrand, catalog number: 08-940)
14. Insulin syringe (BD, catalog number: 14-829-1D)
Note: All surgical instruments should be autoclaved prior to use.
Equipment
1. Centrifuge (Eppendorf, catalog number: 05-413-113)
2. Microwave ablation device (Vision-China Medical Devices R&D center, catalog number: MTC-3C)
3. Water bath (37 °C) (Thermo Scientific, catalog number: 1184L86)
4. Liquid nitrogen tank (Thermo Scientific, catalog number: 31588)
5. Refrigerator (2–8 °C) (Haier, catalog number: HLR-310F)
6. Freezer (-20 °C) (Media, catalog number: MDRS712FIE61W)
7. CO2 incubator (37 °C) (Thermo Scientific, catalog number: 51033555)
8. Biological safety cabinet (Thermo Scientific, catalog number: 1337)
9. Inverted microscope (Olympus, catalog number: IX85P1ZF)
Procedure
文章信息
稿件历史记录
提交日期: Jul 29, 2025
接收日期: Sep 9, 2025
在线发布日期: Sep 17, 2025
出版日期: Oct 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Cao, B., Wheeler, G. L., Mast, J., Zhao, Q. and Shen, J. (2025). Combining Microwave Ablation With CAR-T-Cell Therapy in Tumor-Bearing Mouse Models. Bio-protocol 15(20): e5470. DOI: 10.21769/BioProtoc.5470.
分类
癌症生物学 > 通用技术 > 癌症治疗
癌症生物学 > 肿瘤免疫学 > 癌症治疗
免疫学 > 免疫疗法 > CAR-T
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link