- EN - English
- CN - 中文
Standardized Culture of Skin Fibroblasts From Punch Biopsies for Germline DNA Isolation in Myeloid Malignancies: A Practical Bedside-to-Laboratory Approach
髓系肿瘤中用于生殖系 DNA 分离的皮肤成纤维细胞标准化培养:一种从床旁到实验室的实用方法
(*contributed equally to this work) 发布: 2025年10月05日第15卷第19期 DOI: 10.21769/BioProtoc.5469 浏览次数: 1418
评审: Anonymous reviewer(s)
Abstract
Inherited germline variants are now recognized as important contributors to hematologic myeloid malignancies, but their reliable detection depends on obtaining uncontaminated germline DNA. In solid tumors, peripheral blood remains free of tumor cells and therefore serves as a standard source for germline testing. In contrast, peripheral blood often contains neoplastic or clonally mutated cells in hematologic malignancies, making it impossible to distinguish somatic from germline variants. This unique challenge necessitates using an alternative, non-hematopoietic tissue source for accurate germline assessment in patients with hematologic myeloid malignancies. Cultured skin fibroblasts derived from punch biopsies have long been considered the gold standard for this purpose. Nevertheless, most existing protocols are optimized for research settings and lack detailed, patient-centric workflows for routine clinical use. Addressing this translational gap, we present a robust, enzyme-free protocol for culturing dermal fibroblasts from skin punch biopsies collected at the bedside during routine bone marrow procedures. The method details practical bedside collection, sterile transport, mechanical dissection without enzymatic digestion, plating strategy, culture expansion, and high-yield DNA isolation with validated purity. By integrating this standardized approach into routine hematopathology workflows, the protocol ensures reliable germline material with minimal patient discomfort and a turnaround time suitable for clinical diagnostics.
Key features
• This protocol integrates bedside skin punch biopsy with routine bone marrow sampling to minimize patient discomfort and avoid additional invasive procedures.
• It uses an optimized enzyme-free mechanical dissection method with fat removal, fine mincing, and scratched well plating to reduce contamination and improve fibroblast yield.
• It provides an easy-to-follow workflow for primary fibroblast culture, plating, expansion, and harvest, suitable for routine hematopathology laboratories.
• It consistently yields high-quality germline DNA free of hematopoietic contamination, ideal for genetic testing in myeloid malignancies.
Keywords: Skin fibroblast culture (皮肤成纤维细胞培养)Graphical overview
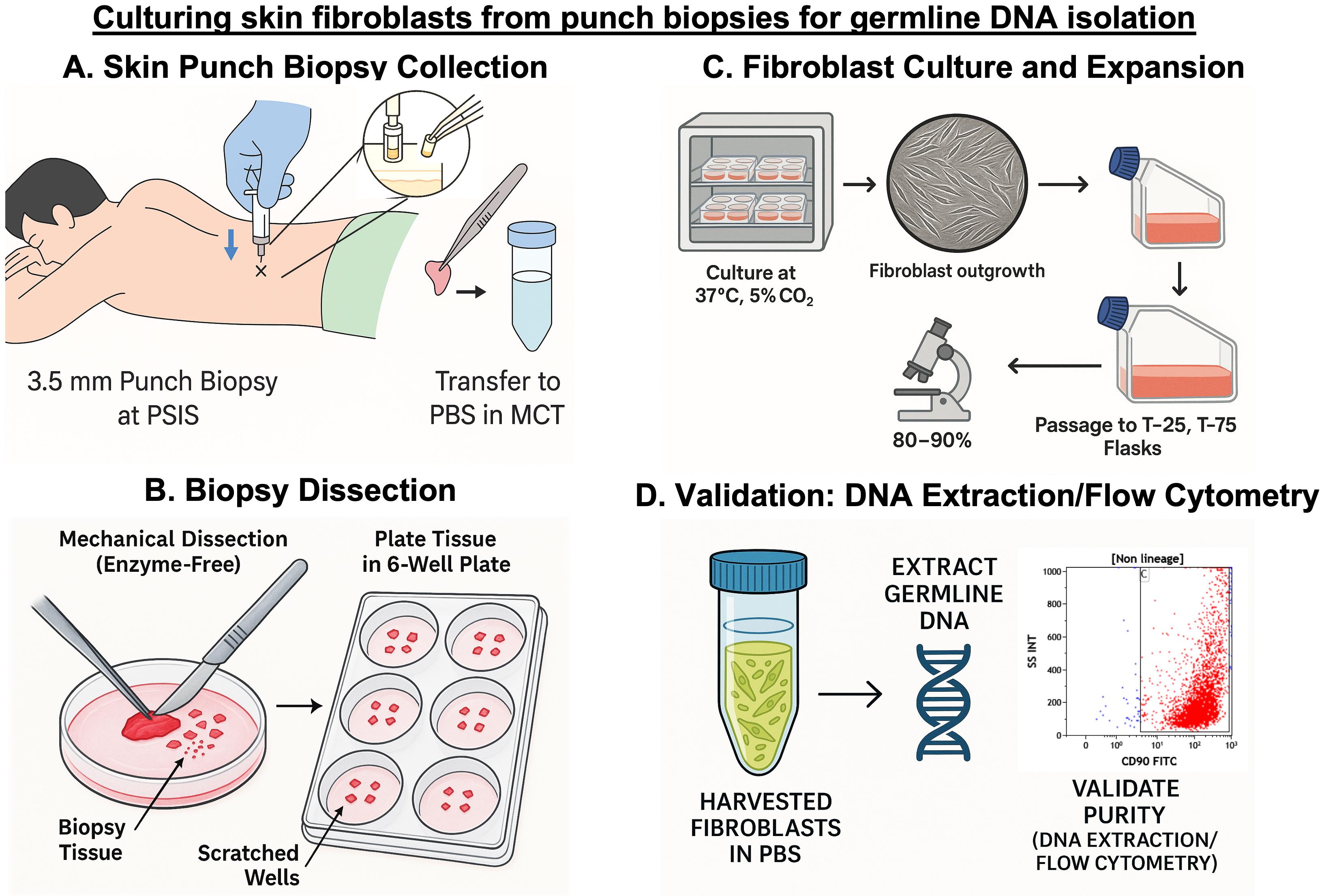
Background
Myeloid malignancies are clonal disorders of hematopoietic progenitor cells characterized by impaired differentiation and deregulated proliferation. These include acute myeloid leukemia (AML), myelodysplastic neoplasms (MDS), myeloproliferative neoplasms (MPN), and overlapping entities such as MDS/MPN [1]. Traditionally, their pathogenesis has been attributed to somatic mutations that perturb essential processes like self-renewal, proliferation, and differentiation [2]. These mutations can be broadly classified into five categories based on function: signal transduction proteins (e.g., JAK2, MPL, CALR, FLT3, and KIT), transcription factors (e.g., CEBPA, RUNX1, and ETV6), epigenetic modifiers (e.g., TET2, DNMT3A, ASXL1, and IDH1/2), tumor suppressors (e.g., TP53, WT1, and PHF6), and splicing factors (e.g., SF3B1, SRSF2, and U2AF1) [3,4]. In recent years, however, there has been increasing recognition that a subset of myeloid neoplasms may arise in the setting of germline predisposition [5–7]. The advent of next-generation sequencing (NGS) has uncovered a growing number of germline variants associated with inherited leukemia predisposition syndromes [8]. The fifth edition of the World Health Organization (WHO) classification of hematolymphoid tumors categorizes such myeloid neoplasms into three groups: (i) those without a pre-existing disorder, including germline mutations in CEBPA and DDX41; (ii) those with pre-existing thrombocytopenia, involving RUNX1, ANKRD26, and ETV6 mutations; and (iii) those with additional organ dysfunction, such as GATA2 deficiency or syndromic disorders like Fanconi anemia, telomere biology disorders, Noonan syndrome, and Down syndrome [1].
Compared to the well-established role of germline variants in solid tumors like breast cancer, our understanding of inherited predisposition in hematologic malignancies remains limited [9]. A unique challenge arises from the fact that several genes implicated in myeloid neoplasms, such as RUNX1, CEBPA, and TP53, can harbor both somatic and germline variants [10,11]. As a result, determining the origin of a detected variant is not always straightforward. In solid tumors, this issue is commonly addressed by paired sampling—comparing the mutation in tumor tissue to that in a non-malignant reference tissue like peripheral blood [12,13]. However, in leukemia and related disorders, both peripheral blood and bone marrow are often involved in the disease process or affected by clonal hematopoiesis, making them unreliable for germline analysis. Consequently, accurate classification of such variants in hematologic malignancies necessitates using alternate, non-hematopoietic tissues.
Among the available non-hematopoietic tissues, cultured skin fibroblasts from punch biopsy specimens have emerged as the most reliable source for germline DNA in patients with hematologic malignancies [14,15]. They offer several advantages, including elimination of contaminating hematopoietic cells, high DNA yield, and reproducibility, making them superior to alternatives such as hair follicles, nail clippings, or buccal mucosa, which often yield inadequate concentration of DNA for clinical-grade assays [14].
While several protocols for culturing fibroblasts from skin biopsies have been published, the majority remain research-oriented and lack direct clinical applicability [16–18]. These protocols often focus on enzymatic digestion techniques or specialized culture conditions, with limited guidance on how they can be effectively integrated into patient care. They also seldom address practical aspects such as bedside sample collection or the need for quick turnaround time in real-world diagnostic settings. To bridge this translational gap, we present a streamlined, enzyme-free protocol specifically optimized for clinical laboratories involved in diagnosing myeloid malignancies. Our method incorporates practical modifications, such as using mechanical separation instead of enzyme-based digestion to separate the epidermis from the dermis, eliminating the need to adhere skin pieces with gelatin, and other simple adjustments that promote faster fibroblast outgrowth. These refinements reduce culture time and contamination risk and are critical when timely and accurate germline results impact patient management. This comprehensive protocol details every step, from bedside collection of the skin punch biopsy alongside routine bone marrow sampling, to standardized fibroblast culture, confluency monitoring, and high-quality DNA extraction for germline variant analysis. The protocol was validated using multiple complementary approaches, including DNA yield assessment, flow cytometry, and immunocytochemistry (ICC). Additionally, the commonly studied JAK2V617F single-nucleotide polymorphism—frequently observed in myeloproliferative neoplasms like polycythemia vera, essential thrombocythemia, and primary myelofibrosis—was specifically used as a prototype during validation. Its detection in the patient’s bone marrow DNA and absence in fibroblast-derived DNA confirmed the lack of hematopoietic contamination and reinforced the reliability of the cultured fibroblasts as a source of pure germline DNA.
By emphasizing feasibility, reproducibility, and clinical relevance, our approach provides a practical framework for integrating fibroblast culture into routine diagnostic workflows for assessing hereditary predisposition in myeloid neoplasms.
Materials and reagents
Biological materials
Human skin punch biopsies were collected from the posterior superior iliac spine of patients diagnosed with various hematological malignancies (n = 18) for protocol validation (Table 1). For standardization, skin biopsies from five patients with non-malignant hematological disorders were used to optimize the method. Biopsies were collected at the same time as routine bone marrow aspiration and trephine biopsy procedures. Written informed consent was obtained from all participants, and the Institutional Ethics Committee approved the study.
Table 1. Clinical details of patients included for protocol standardization and validation
| Patient group | Number of patients | Diagnosis |
|---|---|---|
| Standardization set | 5 | Aplastic anemia (3), immune thrombocytopenia (ITP) (2) |
| Validation set | 18 | Acute myeloid leukemia (AML) (12), myelodysplastic syndrome (MDS) (6) |
Reagents
1. Fetal bovine serum (FBS), heat-inactivated (Gibco, catalog number: 10437028); store at -20 °C for up to 5 years
2. Penicillin-streptomycin (10,000 U/mL) (Gibco, catalog number: 15140122); store at -20 °C for up to 1 year
3. Phosphate buffered saline (PBS), pH 7.2 (Sigma-Aldrich, catalog number: P2272); store at room temperature
4. Dulbecco's modified Eagle medium (DMEM) (Gibco, catalog number: 11995065); store at 4 °C for up to 24 months
5. Trypsin-EDTA (0.25%), phenol red (Gibco, catalog number: 25200056); store at 4 °C for up to 24 months
6. Povidone-iodine solution (10%) (Win Medicare, Betadine 10% solution); store below 22–30 °C, protected from light and moisture
7. Injection lignocaine hydrochloride (2%) (Zydus Healthcare Ltd., Xylocaine 2%); store below 22–30 °C, protected from light and moisture
Solutions
1. 10% complete DMEM (see Recipes)
Recipes
1. 10% complete DMEM
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 1× | 45 mL |
| FBS | 10% | 5 mL |
| Penicillin-Streptomycin | 1% | 0.5 mL |
This should be freshly prepared when required. Before preparation, filter the required volume of DMEM through a 0.2 μm syringe filter and the required volume of FBS through a 0.45 μm syringe filter. After filtering, combine as per the recipe and aliquot into a 50 mL graduated centrifuge sterile tube. Store the freshly prepared complete DMEM at 4 °C.
Laboratory supplies
1. Pipette, 10 μL (RAININ, catalog number: 17014388)
2. Pipette, 200 μL (Sartorius, catalog number: 16598143)
3. Pipette, 1,000 μL (Eppendorf, catalog number: P37952F)
4. Pipette tips, 10 μL (Axygen, catalog number: T-300)
5. Pipette tips, 200 μL (Tarsons, catalog number: 52101Y)
6. Pipette tips, 1,000 μL (Tarsons, catalog number: 521020X-B)
7. Graduated centrifuge tubes, 15 mL (Tarsons, catalog number: 546021)
8. Graduated centrifuge tubes, 50 mL (Tarsons, catalog number: 546041)
9. Gamma-irradiated sterile Petri dish, 90 mm (Abdos Life Sciences, catalog number: P10910)
10. Graduated microcentrifuge tubes, 1.5 mL (Axygen, catalog number: MCT-150-C-S)
11. Graduated microcentrifuge tubes, 2.0 mL (Axygen, catalog number: MCT-200-C-S)
12. Cell culture plate, 6-well, tissue culture treated (Corning, catalog number: 3516)
13. Tissue culture flask, T-75 (Thermo Scientific, catalog number: 156499)
14. Tissue culture flask, 25 cm2, canted neck, 50 mL (Falcon, catalog number: 353108)
15. Sterile single-use hypodermic syringe, 5 mL (DISPO VAN)
16. Sterile single-use hypodermic syringe, 20 mL (DISPO VAN)
17. Sterile single-use needle, 23G × 1” (Safeway)
18. Disposable nitrile gloves (VWR, catalog number: 1122371)
19. Kimwipes (KIMTECH, catalog number: 34155)
20. Syringe filter, 0.45 μm (PALL Life Sciences, catalog number: PN 4614)
21. Syringe filter, 0.2 μm (PALL Life Sciences, catalog number: PN 4612)
22. Parafilm (BEMIS, catalog number: PM-996)
23. Centrifuge tube rack (Falcon or any compatible rack)
24. Sterile gauze (from local vendor)
25. Sterile cotton (from local vendor)
Note: All items that come into contact with biopsy material or cell cultures must be sterile, whether supplied as pre-sterilized single-use consumables or sterilized in-house before use.
Equipment
1. CO2 cell culture incubator (NUAIRE, model: NU-5710E)
2. Biosafety cabinet, Class II Type A2 (ESCO Life Sciences, model: Airstream)
3. Centrifuge, with swing bucket rotor (REMI, model: CM-8 PLUS)
4. Inverted microscope (RADICAL, model: RTC-7)
5. Sterile biopsy punch 3.5 mm diameter, single use (Paramount, catalog number: BPS3.5)
6. Sterile surgical blades No. 22 (GLASS VAN, catalog number: 9512442321)
7. Scalpel handle No. 4, 13.5 cm (GDC, catalog number: 10-100-04E)
8. Straight dissecting forceps, 11.5 cm (from local vendor)
9. Straight pointed scissors, 5" (from local vendor)
10. Sponge holder forceps, 178 mm (from local vendor)
11. Draping sheet (from local vendor)
12. UV light sterilizer bag (from local vendor)
Procedure
文章信息
稿件历史记录
提交日期: Jul 14, 2025
接收日期: Sep 2, 2025
在线发布日期: Sep 17, 2025
出版日期: Oct 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Kour, P., Kumari, N., Kaushal, N., Sharma, T., Naseem, S., Binota, J., Sachdeva, M. S., Jain, A., Rohilla, M., Das, R., Malhotra, P. and Rastogi, P. (2025). Standardized Culture of Skin Fibroblasts From Punch Biopsies for Germline DNA Isolation in Myeloid Malignancies: A Practical Bedside-to-Laboratory Approach. Bio-protocol 15(19): e5469. DOI: 10.21769/BioProtoc.5469.
分类
癌症生物学 > 通用技术 > 分子生物学技术
细胞生物学 > 细胞分离和培养 > 共培养
细胞生物学 > 组织分析 > 组织分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











